5. Special Medical Considerations in FOP
-
5-5. Cardiopulmonary Function in FOP
5-6. Respiratory Health in FOP
5-7. Immunizations in FOP for Diseases Other Than Influenza and Covid-19
5-8. Immunizations for Influenza in FOP
5-9. Coronavirus (COVID-19) Precautions & Guidance for FOP Patients & Families
5-10. Acute & Chronic Pain Management in FOP
5-11. Neurological Issues in FOP
5-12. Developmental Arthropathy and Degenerative Joint Disease in FOP
5-13. Differential Diagnosis of Hip Pain in FOP
5-19. Temporomandibular Subluxations in FOP
5-20. Nutrition, Calcium & Vitamin D Guidelines in FOP
5-21. Preventive Oral Healthcare in FOP
5-22. Extraction of Wisdom Teeth
5-24. Submandibular Flare-ups in FOP
5-26. Dental Anesthesia in FOP
5-27. General Anesthesia in FOP
5-28. Acceptable/Low Risk Procedures in FOP
5-29. Hearing Impairment in FOP
5-30. Gastrointestinal Issues in FOP
5-32. Rehabilitation Issues in FOP
5-33. Aids, Assistive Devices, and Adaptations (AADAs)
5-1. Introduction
Individuals who have FOP can also develop common problems (gall bladder disease, appendicitis, colds, earaches, etc.) as with anyone in the general population. Generally, the safest way to diagnose and treat these problems in a patient with FOP is to ask the question: “How would I evaluate this patient if he or she did not have FOP?” Following that, the “FOP filter” can be applied to ask: “Given the nature of the possible intercurrent medical problem, and the relative risks that particular problem presents in relation to FOP, are there any diagnostic or treatment procedures that should or should not be undertaken (or perhaps alternative diagnostic procedures might be more appropriate)?” Using that approach, diagnostic dilemmas can often be resolved, and appropriate care delivered. When questions remain, experts on FOP should be consulted (Kaplan et al., 2018; see Section 12 – Authors’ Contact Information).
In addition to common medical problems that individuals with FOP might have, there are a number of special medical considerations for FOP patients that are worthy of very special attention. They are presented below.
5-2. Injury Prevention in FOP
Prevention of soft-tissue injury and muscle damage remain a hallmark of FOP management. Intramuscular injections must be avoided. Routine childhood diphtheria-tetanus-pertussis immunizations administered by intramuscular injection pose a substantial risk of permanent heterotopic ossification (HO), as do arterial punctures whereas measles-mumps-rubella immunizations administered by subcutaneous injection and routine venipuncture pose little risk (Lanchoney et al., 1995). Biopsies of FOP lesions are never indicated and will likely cause additional HO. Elective amputations are never indicated.
Permanent ankylosis of the jaw may be precipitated by minimal soft tissue trauma during routine dental care. Assiduous precautions are necessary in administering dental care to anyone who has FOP. Overstretching of the jaw and intramuscular injections of local anesthetic must be avoided. Mandibular blocks cause muscle trauma that will lead to HO, and local anesthetic drugs are extremely toxic to skeletal muscle (Luchetti et al., 1996).
Falls suffered by FOP patients can lead to severe injuries and flare-ups. Patients with FOP have a self- perpetuating fall cycle. Minor soft tissue trauma often leads to severe exacerbations of FOP, which result in HO and joint ankylosis. Mobility restriction from joint ankylosis severely impairs balancing mechanisms, and causes instability, resulting in more falls (Glaser et al., 1998).
Falls in the FOP population are more likely to result in severe head injuries, loss of consciousness, concussions, and neck and back injuries, compared to people who do not have FOP. Individuals with FOP are often unable to use the upper limbs to absorb the impact of a fall. FOP patients are much more likely to be admitted to a hospital following a fall and have a permanent change in physical function because of the fall. In a group of 135 FOP patients, 67% of the reported falls resulted in a flare-up of the FOP. Use of a helmet by young patients may help reduce the frequency of severe head injuries that can result from falls (Glaser et al., 1998).
Measures to prevent falls should be directed at modification of activity, improvement in household safety, use of ambulatory devices (such as a cane, if possible), and use of protective headgear. Redirection of activity to less physically interactive play may also be helpful. Complete avoidance of high-risk circumstances may reduce falls, but also may compromise a patient’s functional level and independence and may be unacceptable to many. Adjustments to the living environment to reduce the number of falls within the home may include installing supportive hand-railings on stairs, securing loose carpeting, removing objects from walkways, and eliminating uneven flooring including doorframe thresholds. Prevention of falls due to imbalance begins with stabilization of gait. The use of a cane or stabilizing device may improve balance for many patients. For more mobile individuals, the use of a rolling cane or a walker will assist in stabilization.
When a fall occurs, prompt medical attention should be sought, especially when a head or neck injury is suspected. Any head or neck injury should be considered serious until proven otherwise. A few common signs and symptoms of severe head injury include increasing headache, dizziness, drowsiness, obtundation, weakness, confusion, or loss of consciousness. These symptoms often do not appear until hours after an injury. An FOP patient should be examined carefully by a healthcare professional if a head or neck injury is suspected.
As mentioned previously, prednisone use should be considered prophylactically following major soft tissue trauma, or peri-operatively. The dose of corticosteroids is dependent upon body weight. A typical dose of prednisone is 1-2 mg/kg/day (up to 100 mg), administered as a single daily dose for no more than 4 days (Table 1). In order to have the least suppressive effect on the hypothalamic-pituitary-adrenal axis, medication should be taken in the morning.
References
Glaser DL, Rocke DM, Kaplan FS. Catastrophic falls in patients who have fibrodysplasia ossificans progressiva. Clin Orthop 346: 110-116, 1998
Lanchoney TF, Cohen RB, Rocke DM, Zasloff MA, Kaplan FS. Permanent heterotopic ossification at the injection site after diphtheria-tetanus-pertussis immunizations in children who have fibrodysplasia ossificans progressiva. J Pediatrics 126: 762-764, 1995
Luchetti W, Cohen RB, Hahn GV, Rocke DM, Helpin M, Zasloff M, Kaplan FS. Severe restriction in jaw movement after route injection of local anesthetic in patients who have fibrodysplasia ossificans progressiva. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 81: 21-25, 1996
5-3. Scalp Nodules in FOP
Scalp nodules are flare-ups on the head, commonly appearing in childhood – often the first post-natal manifestations of FOP. Scalp nodules are of little clinical significance despite their often large size and alarming appearance.
Scalp nodules are noted in very few publications (Kitterman et al., 2005; Piram et al., 2011; Kardile et al., 2012; Al Kaissi et al., 2016). They are often reported as first symptom of FOP, from the neonatal period (about 10% of cases (Kitterman et al., 2005), but also may be an under-recognized symptom at any age. The median age of onset is 1.5 years (Piram et al., 2011).
Clinically, scalp nodules may be solitary or numerous, immobile, with a variable size, from a walnut size to a great volume such as a tennis ball. They may be asymptomatic or painful only at onset. Usually, they appear and regress spontaneously, or can develop after trauma or infection, or in a child who is apparently in good health.
Radiographs are unnecessary but generally show soft tissue thickening initially with small zones of HO after some months. Biopsies/surgical excision or fine-needle aspiration for cytological and histologic studies must be avoided. Histopathologic findings are described in three patients by Piram et al., 2011 as proliferation of short spindle-shaped cells in the deep subcutaneous tissue, with abundant collagenous stroma and scattered inflammatory cells (mast cells & T-cells) and numerous vessels.
The correlation between their presence and the genotype is not detailed in the few reported cases but it appears that they are associated with the R206H classic mutation in ACVR1 in numerous cases studied.
The occurrence of unique or multiple scalp nodule(s) in infancy is an important early sign of FOP and may be the first sign of a post-natal flare-up of the condition. The presence of scalp nodules during infancy should prompt immediate examination of the great toes – a clinical scenario that could appropriately accelerate the proper diagnosis of FOP.
So, importantly, when scalp nodules are seen, the toes must be examined! Even more importantly, biopsies must not be performed. Symptomatic treatments may be prescribed but steroids are not used as no joints are involved. Despite their often alarming appearance, no treatment is necessary. Swelling subsides with time – and if ossification occurs, remodeling is common as the lesions are incorporated into the growing skull.
References
Al Kaissi A, Kenis V, Ben Ghachem M, Hofstaetter J, Grill F, Ganger R, Kircher SG. The diversity of the clinical phenotypes in patients with fibrodysplasia ossificans progressiva. J Clin Med Res 8: 246-253, 2016
Kitterman JA, Kantanie S, Rocke DM, Kaplan FS. Iatrogenic harm caused by diagnosis errors in FOP. Pediatrics 116: 654-661, 2005
Kardile M, Nayak S, Nagaraja HS, Mishra AK. Fibrodysplasia ossificans progressiva in a four-year-old child. J Orthop Case Rep 2: 17-20, 2012
Piram M, Le Merrer M, Bughin V, De Prost Y, Fraitag S, Bodemer C. Scalp nodules as a presenting sign of fibrodysplasia ossificans progressiva: a register-based study. J Am Acad Dermatol 64: 97-101, 2011
5-4. Spinal Deformity in FOP
Spinal deformities are common in individuals who have FOP. A study in 40 FOP patients showed that 65% had radiographic evidence of scoliosis. The initial clinical abnormality was a rapidly developing scoliosis associated with a spontaneously occurring lesion in the paravertebral soft tissues. Once established, these deformities lead to rapid, permanent loss of mobility and to progressive spinal deformity with growth (Shah et al., 1994).
The formation of a unilateral osseous bridge along the spine prior to skeletal maturity limits growth on the ipsilateral side of the spine while growth continues uninhibited on the contralateral side. If an osseous bridge occurs bilaterally and the two bridges are relatively symmetrical, or if an osseous bridge forms after skeletal maturity, scoliosis will not result.
Severe scoliosis in FOP can lead to pelvic obliquity, similar to that seen in scoliosis resulting from other causes, and the obliquity can impair the balance of the trunk as well as standing and/or sitting balance.
Anecdotal experience in five patients suggests that traditional operative approaches to scoliosis in FOP patients can seriously exacerbate the disease. Furthermore, three patients in the series who had operative correction of the scoliosis continued to have progression of the spinal curve even after a spinal arthrodesis. In two of these patients, the arthrodesis was performed posteriorly and not anteriorly. Thus, continued anterior growth of the spine exacerbated rotational deformity.
Indications for correction of spinal deformity associated with more usual types of scoliosis do not pertain to patients with FOP. With the limited knowledge available, the risks of severe complications (most notably, the exacerbation of HO at sites remote from the operative field) that are associated with correction of spinal deformity in FOP likely outweigh the benefits (Shah et al., 1994). However, with greater knowledge of the natural history of FOP and newer surgical techniques, these old assertions are undergoing careful re-examination on a case-by-case basis.
A study of three patients with rapidly evolving chin-on-chest deformities suggests that a more aggressive surgical approach may be necessary to prevent and/or correct such rapidly progressive deformities in patients who have FOP (Moore et al., 2009).
References
Moore R, Dormans J, Drummond DS, Shore EM, Kaplan FS, Auerbach J. Chin-on-chest deformity in patients who have fibrodysplasia ossificans progressiva (FOP). J Bone Joint Surg Am 91: 1497-1502, 2009
Shah P, Zasloff MA, Drummond, D, Kaplan FS. Spinal deformity in patients who have FOP. J Bone Joint Surg Am 76: 1442-1450, 1994
5-5. Cardiopulmonary Function in FOP
A recent natural history study showed that individuals with ACVR1R206H mutations have an increased prevalence of cardiac conduction abnormalities on electrocardiogram. Conduction abnormalities were present in 45.3% of baseline ECGs, with the majority of abnormalities classified as nonspecific intraventricular conduction delay (37.7%). More specifically, 22.2% of patients > 18 years old had conduction abnormalities, which was significantly higher than a prior published study of a healthy population (5.9%; n = 3978) (p < 0.00001). Patients with FOP < 18 years old also had a high prevalence of conduction abnormalities (62.3%). The 12-month follow up data was similar to baseline results. Conduction abnormalities did not correlate with chest wall deformities, scoliosis, pulmonary function test results, or increased Cumulative Analog Joint Involvement Scale (CAJIS) scores. Echocardiograms from 22 patients with FOP revealed eight with structural cardiac abnormalities, only one of which correlated with a conduction abnormality (Kou et al., 2020).
In summary, individuals with FOP may have subclinical conduction abnormalities manifesting on ECG, independent of heterotopic ossification. Although clinically significant heart disease is not typically associated with FOP, and the clinical implications for cardiovascular risk remain unclear, knowledge about ECG and echocardiogram changes is important for clinical care and research trials in patients with FOP. Further studies on how ACVR1/ALK2R206H affects cardiac health will help elucidate the underlying mechanism (Kou et al., 2020).
Patients with FOP develop thoracic insufficiency syndrome (TIS) that can lead to life-threatening complications. Features contributing to TIS in patients with FOP include:
Costovertebral malformations with orthotopic ankylosis of the costovertebral joints
Ossification of intercostal muscles, paravertebral muscles and aponeuroses
Progressive spinal deformity including kyphoscoliosis or thoracic lordosis
Pneumonia, hypoxemia, hypercarbia, pulmonary hypertension and right-sided heart failure are the major life-threatening hazards that result from TIS in patients with FOP. Prophylactic measures to maximize pulmonary function, minimize respiratory compromise, and prevent influenza and pneumonia are helpful in decreasing the morbidity and mortality from TIS in patients with FOP (Kussmaul et al., 1998; Kaplan & Glaser, 2005; Kaplan et al., 2010). A pulmonologist should be involved early with regular spirometry assessments and sleep studies as needed.
Individuals with FOP develop progressive limitations in chest expansion, resulting in restrictive lung disease, with reduced vital capacity but no obstruction to air flow. Those with advanced disease have extremely limited chest expansion and rely on the diaphragm for inspiration (Kussmaul et al., 1998). The low inspiratory capacity results in low expiratory flow rates, in many cases.
Consequently, individuals with FOP are subject to atelectasis, retained secretions, hypoxemia, hypercarbia and pneumonia. All patients had abnormal spirometry secondary to TIS. Chest infection in the presence of diminished pulmonary reserve is the major hazard to life in patients with FOP. Many patients had abnormal electrocardiograms, with evidence of right ventricular dysfunction. It is suggested that the presence of severe restrictive disease of the chest wall is associated with a high incidence of right ventricular abnormalities (Kaplan et al., 2010).
Respiratory failure and cor pulmonale are features of severe TIS (Shah et al., 1974). A detailed description of this problem (Bergofsky et al., 1979) noted right ventricular hypertrophy in at least 10% of cases.
Pulmonary hypertension was a common finding, which these authors attributed to increased vascular resistance and the effects of prolonged alveolar hypoventilation.
The respiratory problems seen in patients with FOP are similar to those seen in patients with respiratory muscle weakness such as cervical spinal cord injury, or other skeletal abnormalities such as kyphoscoliosis. Strategies similar to those used in these other populations to maximize respiratory muscle functional and clear secretions may be beneficial in those with FOP.
Inspiratory and expiratory muscle training should be routinely practiced and started at the age of diagnosis. A variety of incentive spirometers are available to encourage deep breathing. Inspiratory muscle training devices permit progressive resistance exercise training of the diaphragm.
Careful attention should be directed toward the prevention and therapy of intercurrent chest infections. Such measures should include prophylactic pneumococcal pneumonia and influenza vaccinations (given subcutaneously), chest physiotherapy, and prompt antibiotic treatment of early chest infection.
Upper abdominal surgery should be avoided, if possible as it interferes with diaphragmatic respiration. Sleep studies to assess sleep apnea may be helpful. Positive pressure assisted breathing devices such as BiPAP® (Bi-level positive airway pressure) masks without the use of supplemental oxygen may also be helpful. Night CPAP and noninvasive ventilation on ambient air may be effective treatments for nocturnal hypoventilation/hypoxemia.
Patients with FOP who have advanced TIS and who use unmonitored oxygen have a high risk of sudden death. Sudden correction of oxygen tension in the presence of chronic carbon dioxide retention suppresses respiratory drive. Patients who have FOP and severe TIS should not use supplemental oxygen in an unmonitored setting (Kaplan & Glaser, 2005; Kaplan et al., 2010).
During hospitalizations or in more advanced disease, individuals with FOP may have trouble clearing secretions. This can lead to atelectasis, pneumonia and respiratory failure requiring endotracheal intubation. Secretion clearance is enhanced by adequate hydration, guaifenesin, bronchodilators and mucolytics, as needed. If endotracheal intubation or a surgical procedure are considered, nasal fiberoptic intubation of the trachea is recommended (Kilmartin et al. 2014). For surgical or interventional procedures, a carefully designed anesthesia plan is paramount. Planning for extubation of the trachea should be weighed against creation of tracheostomy if impending respiratory failure is expected due to the advanced TIS. Postoperative care should be assigned to an intensive care setting.
There is also much that can be done in prevention. Individuals with FOP are often born with congenital malformations of the costovertebral joints that cause some degree of chest restriction even before the appearance of heterotopic bone, although these restrictions may not lead to any clinical problems early in life. However, because of these restrictions, individuals with FOP are more likely to rely, even early in life, on diaphragmatic breathing. It is recommended that individuals with FOP be evaluated by a pulmonologist by the end of the first decade of life in order to perform baseline pulmonary function tests and echocardiograms. The results of these tests may further help guide preventative care for the cardiopulmonary system.
Several devices are available to loosen secretions from relatively simple handheld devices that cause vibration of the airway walls during exhalation, to garments that vibrate the chest wall to high technology specialty beds that turn and oscillate. Care must be taken when using such devices in patients with a weak cough, as they may be unable to expectorate the secretions once loosened. Use of mechanical insufflation-exsufflation can non-invasively extract retained secretions from individuals with ineffective cough. The device can dramatically increase peak cough expiratory flow in individuals with impaired expiratory muscle function. Combining a method to loosen secretions with in-exsufflation to remove them may prevent respiratory failure and the need for mechanical ventilation. However, all percussive devices should be used with caution due to risk of inducing trauma.
Various activities can help maximize the strength of the diaphragm and perhaps decrease the risk of intercurrent pulmonary problems. In addition to the intermittent use of incentive spirometry, other activities such as deep breathing, swimming/hydrotherapy, and singing, should be encouraged at an early age and may help improve long-term pulmonary function.
Pulmonary hypertension (PH) is a complication of TIS which is a common feature of FOP. Early symptoms are non-specific and include dyspnea on exertion and fatigue. In FOP, a high index of suspicion is warranted if severe restrictive lung disease or moderate disease exists over a prolonged period of time. If PH is suspected transthoracic echocardiography should be performed, since it permits a noninvasive estimation of pulmonary arterial systolic pressure (usually > 25 mm Hg in PH) and evaluation of both right and left heart size and function. Although right heart catheterization confirms the diagnosis of PH, it can reasonably be deferred in FOP in the setting of known severe restrictive lung disease or other proximal causes such as advanced left-sided heart disease or chronic hypoxia. When possible, treatment of the underlying condition is the primary goal of management for PH; however, in FOP, PH is most commonly associated with restrictive lung disease which is not easily treated due to immobility of the chest cavity. Therefore, in FOP, therapy for PH is directed at PH itself and is best managed by pulmonologists.
Anecdotal evidence exists for the use of phosphodiesterase-5 inhibitors such as sildenafil or tadalafil, and should be considered as an initial oral therapy for mild to moderate PH.
References
Bergofsky EH. Respiratory failure in disorders of the thoracic cage. Am Rev Resp Dis 119: 643–669, 1979
Kaplan FS, Glaser DL. Thoracic insufficiency syndrome in patients with fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab 3: 213-216, 2005
Kaplan FS, Zasloff MA, Kitterman JA. Shore EM, Hong CC, Rocke DM. Early mortality and cardiorespiratory failure in patients with fibrodysplasia ossificans progressiva. J Bone Joint Surg Am 92: 686-691, 2010
Kilmartin, E, Grunwald, Z, Kaplan FS, Nussbaum BL. General anesthesia for dental procedures in patients with fibrodysplasia ossificans progressiva: A review of 42 cases in 30 patients. Anesth Analg 118: 298- 301, 2014
Kou S, De Cunto C, Baujat G, Wentworth KL, Grogan DR, Brown MA, Di Rocco M, Keen R, Al Mukaddam M, le Quan Sang KH, Masharani U, Kaplan FS, Pignolo RJ, Hsiao EC. Patients with ACVR1(R206H) mutations have an increased prevalence of cardiac conduction abnormalities on electrocardiogram in a natural history study of fibrodysplasia ossificans progressiva. Orphanet J Rare Dis 2020 Jul 29;15(1):193
Kussmaul WG, Esmail AN, Sagar Y, Ross J, Gregory S, Kaplan FS. Pulmonary and cardiac function in advanced fibrodysplasia ossificans progressiva. Clin Orthop 346: 104-109, 1998
Shah PB, Zasloff MA, Drummond D, Kaplan FS. Spinal deformity in patients who have fibrodysplasia ossificans progressiva. J Bone Joint Surg Am 76: 1442–1450, 1994
5-6. Respiratory Health in FOP
Strong respiratory health is important for everyone. This is particularly true for individuals with FOP since FOP can severely decrease respiratory capacity from the chest wall deformities, heterotopic ossification and scoliosis (Shah et al.,1994; Towler et al., 2020; Botman et al, 2021).
Maintaining strong respiratory health involves a few things:
Infection precautions, particularly during the flu season: Make sure that all FOP patients and their family members wash their hands regularly and use alcohol gel. Avoid places where infection can be easily transmitted. In the event that exposures need to occur, we recommend that individuals with FOP wear a simple surgical mask to decrease the risk of breathing in infected droplets (such as from a sneeze). These masks are not meant to filter out all viruses and infectious bacteria but will balance the need to decrease exposure to larger droplets with comfortable breathing. Alternatively, a high quality N95 or KN95 mask can be very effective in decreasing exposure.
Maintaining respiratory capacity: We recommend 15-30 minutes per day of active respiratory activity. This should not be uncomfortable or cause pain but is meant to help keep the diaphragm and other respiratory muscles healthy. Activities that we recommend include vigorous vocalizations (i.e. singing, as in a choir; loud continuous vocal activity like singing in the shower), blowing bubbles, etc.
For some, an incentive spirometer can complement vigorous vocalizations. These devices can be used to measure lung capacity. However, we recommend using them as a way to maintain lung capacity and make sure the lungs are well-ventilated.
FOP patients who decide to have yearly influenza immunizations should use the subcutaneous immunization, given by an experienced provider. The flu immunization should not be given during a flare- up and not until at least 2 weeks after a flare-up has resolved. Whether or not the FOP patient decides to get a flu vaccine, all immediate family members, household contacts and caretakers should be immunized.
In the case where the flu vaccine is not obtained, for whatever reason, having a ready supply of Oseltamivir (Tamiflu) on hand during flu season is a reasonable precaution. At the first signs of the flu (fever or feeling feverish/chills, cough, sore throat, runny or stuffy nose, muscle or body aches, headaches, fatigue (tiredness), vomiting and diarrhea), one should take the first dose of Oseltamivir and then immediately contact their physician (Jefferson et al., 2014).
Patients should also consider vaccines for other respiratory illnesses, including pneumococcus and COVID-19. See the respective sections for additional details.
There are many types of incentive spirometers available and many different strategies for maintaining lung function. The IFOPA provides two types depending on age and jaw needs.
Peak Flow Whistle (for young children)
For young children, a peak flow whistle is recommended. These whistles will make a sound when air is blown fast enough through the whistle. The most important part of this device is taking the deep breath beforehand – not the actual ability to generate the whistle sound!
The patient should:
Sit upright or stand.
Place the whistle in the mouth, and make sure the lips are tightly sealed
Slowly inhale as much as possible (this is the most important part). Hold the breath for about 10 seconds.
Exhale quickly through the whistle to generate the sound.
Rest in between breaths.
Repeat 10 times, with short rest breaks in between.
The patient should stop if they feel dizzy at any time, or if they have any tenderness or chest discomfort.
The whistle should be set based on the patient’s estimated peak flow on a regular day (i.e. should just be able to whistle). Standard tables with FEV1 values are not useful in FOP due to the presence of chest deformities; however, prior PFT values can serve as a guide. The goal is to encourage deep breaths to minimize atelectasis, rather than increasing peak flow. The flow whistle serves as an incentive for children.
Incentive Spirometer
The incentive spirometer is for older children and adults. There are many models available. The goal, however, is the same with all the models - to take slow, deep breaths to expand the lungs.
The patient should:
Sit upright in a chair or bed or stand upright.
Hold the spirometer in front of the face at eye level. Many FOP patients will not be able to do this due to ankylosis of the joints in the upper extremities. They may need assistance or have the spirometer positioned close enough to use but without having to hold with both hands.
Close your lips around the mouthpiece to make a seal.
Slowly exhale completely.
Slowly inhale thorough the mouth as deeply as possible.
The piston will rise with inhalation.
Hold the breath for 10 seconds (the piston may fall during this time), then exhale.
Repeat 10 times, with short rest breaks in between.
Stop and rest if they feel dizzy at any time.
Perform this routine twice daily.
References
Botman E, Smilde BJ, Hoebink M, Treurniet S, Raijmakers P, Kamp O, Teunissen BP, Bökenkamp A, Jak P, Lammertsma AA, van den Aardweg JG, Boonstra A, Eekhoff EMW. Deterioration of pulmonary function: An early complication in Fibrodysplasia Ossificans Progressiva. Bone Rep 2021 Feb 25;14:100758
Jefferson T, Jones MA, Doshi P, Del Mar CB, Hama R, Thompson MJ, Spencer EA, Onakpoya I, Mahtani KR, Nunan D, Howick J, Heneghan CJ. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev 2014 Apr 10;(4)
Shah PB, Zasloff MA, Drummond D, Kaplan FS. Spinal deformity in patients who have fibrodysplasia ossificans progressiva. J Bone Joint Surg 76-A: 1442-1450, 1994
Towler OW, Shore EM, Kaplan FS. Skeletal malformations and developmental arthropathy in individuals who have fibrodysplasia ossificans progressiva. Bone 2020 Jan;130:115116.
5-7. Immunizations in FOP for Diseases Other Than Influenza and Covid-19
Vaccines against various infectious diseases have dramatically decreased morbidity and mortality from infectious diseases (Roush et al., 2007). Because individuals with FOP are subject to the same infectious diseases as the general population, immunizations are essential in FOP. However, there are several major considerations and precautions regarding immunization of those with FOP, and these are discussed below.
The Advisory Committee on Immunization Practices (ACIP) of the Center for Disease Control and Prevention (CDC) has published charts with their recommendations for immunization for children, adolescents, and adults. The chart for children and adolescents can be found at: https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html
The chart for adults is at: https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html
In addition, these sites contain a wealth of other information about immunizations including Parent Friendly Immunization Charts, Resources for Parents, Resources for Adults, Resources for Health Professionals, Vaccine Information Statements, and up to date clinical information about Covid-19 vaccines. Hard copies of the Immunization Charts can be obtained for free using these sites.
Those immunization recommendations, last updated in February 2023, have been approved by the American Academy of Pediatrics, the American Academy of Family Practitioners, and the American College of Obstetricians and Gynecologists.
For most of the vaccines given in childhood, the ACIP recommends intramuscular (IM) administration. However, IM injections are contraindicated in FOP because of the risk of heterotopic ossification (HO) at the injection site and sometimes elsewhere in the body. Lanchoney and associates reported that IM injection of diphtheria-pertussis-tetanus (DPT) vaccines caused flare-ups and subsequent HO in 27% of children with FOP and, in some cases, permanent loss of joint motion (Lanchoney et al., 1995).
Furthermore, subcutaneous (SubQ) injection of DPT type vaccines may also cause flare-ups, HO and loss of joint mobility (F. Kaplan, personal communication). Therefore, it seems that some unidentified component(s) of DPT type vaccines may cause flare-ups and subsequent HO in individuals with FOP regardless of route of administration. Given these experiences, it is recommended that no DPT type vaccines be given to individuals with FOP.
Other immunizations given by the SubQ route have not been reported to cause flare-ups or HO in those with FOP. Specifically, there are no reported cases of flare-ups following subcutaneous immunization with the MMR or MMRV vaccines despite the fact that they contain live viruses.
In individuals with hemophilia, IM injections may cause hemorrhage. Because of this risk, the World Federation for Hemophilia recommends the SubQ route for all immunizations for individuals with hemophilia (Srivastava et al., 2020). It is standard practice at most Hemophilia Treatment Centers to recommend SubQ administration of all vaccines (Ragni et al., 2000; Ritchey, 2005; Carpenter et al., 2015; Schaefer et al., 2017). However, of the vaccines recommended for IM administration and given SubQ to hemophilia patients, to date only Hepatitis A (Ragni et al., 2000), Hepatitis B (Carpenter et al., 2015), and diphtheria-tetanus (Cook, 2008; Schaefer et al., 2017) vaccines have been shown to be effective in providing immunity. There are no published data regarding effectiveness of other IM vaccines given SubQ.
A concern for giving several vaccines SubQ has been granuloma formation at the injection site (Pembroke & Marten, 1979). These granulomas are considered to be the result of hypersensitivity to aluminum, an adjuvant to the other components of the vaccine. In a prospective cohort study in Sweden, Bergfors and associates reported that long lasting, intensely itching granulomas occurred in less than 1% of children receiving injections of DPT vaccines. The incidence of granulomas was similar whether the injections were given IM or SubQ (Bergfors et al., 2003 and 2014). Furthermore, granulomas have not been reported with SubQ vaccines administered to patients with hemophilia (Ritchey, 2005; J. Huang, personal communication). We are not aware of granuloma formation after any immunizations in individuals with FOP.
Based on the above information, it may seem reasonable to recommend that individuals with FOP receive all their recommended immunizations by SubQ injection. However, the situation is more complicated than that. Several of the routine vaccines are conjugated with components of either diphtheria or tetanus vaccines. Because the component(s) in DPT type vaccines that cause FOP flare-ups and HO have not been identified, it may be prudent for FOP patients to avoid vaccines that are conjugated with components of DPT vaccines (See Sections 4 and 5 below).
Recommendations for Immunization in FOP:
The following sections list vaccines recommended by the ACIP for routine immunization of individuals from birth to 18 years of age and for adults along with our cautions regarding administration to individuals with FOP. The median age of diagnosis of FOP is just under six years (Kitterman et al., 2005). Therefore, many vaccines will have already been administered IM by the time that the person has been diagnosed with FOP.
General Recommendation:
Do not give ANY immunizations to individuals with FOP during a flare- up. It would be preferable to wait 6 to 8 weeks after the flare-up has clinically resolved.
Recommendations Regarding Specific Vaccines:
1. Vaccines recommended by the ACIP to be given SubQ to all and appear to be safe for FOP patients: (These contain no components of diphtheria or tetanus.)
Measles, Mumps, Rubella Vaccine (MMR; brand names: M-M-R II, Priorix)
Varicella Vaccine (VAR; brand name: Varivax)
Measles, Mumps, Rubella, Varicella Vaccine (MMRV; brand name: ProQuad)
Inactivated Polio Vaccine (IPV; brand name: Ipol)
Pneumococcal 23-valent Polysaccharide Vaccine (PPSV23; brand name: Pneumovax-23)
2. Vaccines recommended by the ACIP to be given IM but are effective SubQ and are probably safe for FOP patients: (These contain no components of diphtheria or tetanus.)
Hepatitis A Vaccine (HepA; brand names: Havrix, VAQTA)
Hepatitis B Vaccine (HepB; brand names: Energix-B, Recombivax-HB, Heplisav-B)
3. Vaccines recommended by the ACIP to be given IM and are probably safe for FOP patients, but there are no data on their effectiveness when given SubQ: (These contain no components of diphtheria or tetanus.)
Meningococcal serogroup B Vaccine (MenB; brand names: Bexsero; Trumenba)
Human Papilloma Virus Vaccine (HPV; brand name:Gardisil-9)
Haemophilus influenzae type B vaccine (HiB: brand name: PedvaxHIB). Note that this is the only brand of HiB that is not conjugated to a component of diphtheria or tetanus.
4. Vaccines recommended by the ACIP to be given IM, but there are no data on their effectiveness when given SubQ, and these vaccines may NOT be safe for FOP patients because they are conjugated to a component of diphtheria or tetanus:
Haemophilus influenzae type B vaccine (HiB; brand names: Hiberix; Act HiB)
Meningococcal serogroups A, C, W, Y (brand names: Menactra; Menveo)
Pneumococcal 13-valent conjugate vaccine (PCV13; brand name: Prevnar-13)
Pneumococcal 15-valent conjugate vaccine (PCV15; brand name: Vaxneuvance)
HiB/MenC combination vaccine (brand names: Menitorix; Menhibrix)
5. Diphtheria, Pertussis, Tetanus type Vaccines:
These vaccines are not routinely recommended in FOP because of the experience that they may cause flare- ups, HO, and permanent loss of joint motion (Lanchoney et al., 1995; F. Kaplan, personal communication). Therefore, certain precautions should be taken to avoid or treat the diseases that these vaccines prevent.
However, there may be certain situations where the vaccine needs to be given to prevent life threatening illness (See Section C 4, below):
a. Diphtheria is a rare disease in the United States with less than 1 case per year in the past 20 years. If diphtheria is suspected clinically, follow the diagnosis and treatment guidelines of the CDC (https://www.cdc.gov/diphtheria/index.html) and the American Academy of Pediatrics (Kimberlin et al., 2021, Diphtheria) and consult with an infectious disease specialist.
b. Pertussis is a particular risk for infants and those with respiratory compromise (common in FOP). All household contacts of individuals with FOP should be immunized for pertussis. If there is a local outbreak of pertussis, those with FOP should not attend school during the outbreak. If pertussis is suspected in an individual with FOP, start early treatment with antibiotics as recommended by the CDC (https://www.cdc.gov/pertussis/) and the American Academy of Pediatrics (Kimberlin et al, 2021, Pertussis). If a household contact is diagnosed with pertussis, the individual with FOP should be given post-exposure prophylaxis.
c. Tetanus. In case of a wound considered a risk for tetanus, follow the guidelines for “Wound Management for Tetanus Prevention” of the CDC (https://www.cdc.gov/tetanus/index.html) and the American Academy of Pediatrics (Kimberlin et al, 2021, Tetanus). In addition, it is recommended that consultation be obtained with an infectious disease specialist. The use of Tetanus Immune Globulin (TIG) given subcutaneously should be considered for acute management.
Additional considerations about TIG for FOP include:
1. If TIG is to be given IM, the injection site should be chosen to be near joints or muscles that have already lost function. This may be a non-standard site. Thus, if a flare-up develops, it is less likely to worsen mobility significantly. Alternatively, TIG can be given SubQ, but the efficacy is unknown. However, recent data in immunocompromised patients show that immunoglobulins can be effectively delivered SubQ.
2. Because IM injection of TIG may precipitate a flare-up, give prophylactic prednisone 2 mg/kg/day (up to a maximum of 100 mg/day) for 2 days. Then taper the daily dose by 50% every other day. Note that this is shorter than the usual course for treatment of a flare-up, which is usually 4 days at 2 mg/kg/day. Prophylactic prednisone is recommended only with TIG and is not necessary for other immunizations.
3. Because the IM injection can cause significant inflammation, give ibuprofen immediately prior to the injection and continue with standard age/weight appropriate dosing for 7 days, even if no symptoms are present.
4. In the event that TIG is not available, and the only option is to use a Td containing vaccine, the Td vaccine should be given SubQ and the patient with FOP should be given prophylactic prednisone 2 mg/kg/day (up to a maximum of 100 mg/day) for 2 days. Then taper the daily prednisone dose by 50% every other day. Note that this is shorter than the usual course for treatment of a flare-up, which is usually 4 days at 2 mg/kg/day followed by a daily taper.
6. Rotavirus Vaccines (brand names: Rotarix, RotaTeq) are oral vaccines that contain live virus. The CDC recommends these vaccines for infants 2 to 6 months of age to prevent gastroenteritis caused by Rotavirus. Because the majority of those with FOP are not diagnosed until later in life, many with FOP will already have received one of these vaccines before FOP has been diagnosed.
7. Dengue Vaccine (brand name: Dengvaxia) is recommended only for children and adolescents 9- 16 years old who have laboratory-confirmed previous dengue virus infection and are living in an area where dengue is endemic.
8. Other Vaccines
a. Covid-19 Vaccines. See section on COVID vaccines
b. Cholera Vaccine (brand names: Dukoral; Shancol; Euvichol-Plus): These oral vaccines are recommended only for the following: (i) in areas where local transmission of cholera occurs; (ii) during humanitarian crises with a high risk of cholera; and (iii) during outbreaks of cholera. None of these vaccines are currently available in the United States. No data are available regarding these vaccines in FOP. (https://www.cdc.gov/cholera/vaccines.html)
c. Japanese Encephalitis Vaccine (JE-Vax; brand name: Ixiaro) is routinely given in several Asian countries where there is a high risk of this disease. This vaccine is given IM; there is no information regarding SubQ administration of Ixiaro. Because of the high rates of mortality and neurologic sequelae from Japanese Encephalitis, (Hills et al, 2019), individuals planning to visit Asia should discuss with their physician whether the protective effect of the vaccine outweighs the risk of flareups or HO from the IM administration of the vaccine.
d. Rabies Vaccine (brand names: Imovax; RabAvert) are cell culture vaccines that are recommended by the CDC and are administered on days 0, 3, 7, and 14 after exposure to Rabies (https://www.cdc.gov/vaccines/hcp/vis/vis-statements/rabies.html). Both vaccines can be given via the intradermal route which is as immunogenic and safe as IM (https://www.who.int/teams/control-of-neglected-tropical-diseases/rabies/vaccinations-and- immunization).
e. Tuberculosis: Bacille Calmette-Guerin (BCG) Vaccine is routinely given to infants to prevent tuberculosis in countries and areas where there is a high risk for the disease. Depending on the preparation, BCG can be given percutaneously with a multiple puncture device (https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm20 2934.pdf) or injected intradermally (Hawkridge et al., 2008).
f. Typhoid Fever vaccine (brand names: Typhim Vi; Vivotif): The World Health Organization (WHO) recommends immunization for protection against Typhoid Fever only for individuals in areas where there is a high risk for the disease. Typhim Vi is given IM for individuals >2 years of age; Vivotif is given orally for individuals >6 years of age. Individuals with FOP who plan to travel to areas where there is a high risk of Typhoid Fever should consult with their physician to decide whether the risk of Typhoid Fever is greater than the risk of the vaccine. Two other typhoid vaccines (Typbar Vi and Pedatyph) are conjugated to tetanus toxoid and should not be given to individuals with FOP.
g. Yellow Fever Vaccine (brand names: YF-VAX, Stamaril). YF-VAX is recommended for persons age 9 months and older who are traveling to or living in areas at risk for Yellow Fever transmission in South America and Africa. This vaccine is given SubQ. At this time there is limited availability of YF-VAX. Stamaril is not available in the United States.
h. Zoster Vaccines (brand names: Shingrix; Vostarax) The ACIP recommends Zoster Vaccine only for individuals 50 years of age and older to prevent Shingles (Herpes Zoster), which is due to reactivation of the Zoster-Varicella virus that causes Chickenpox. There are two vaccines available. Shingrix is a recombinant vaccine that contains no live virus. Vostarax is a live virus vaccine. For individuals with FOP 50 years of age and older, these vaccines should be given SubQ.
i. Respiratory Syncytial Virus (RSV). At this time there are two approved vaccines (Arexvy or Abrysvo) for RSV. Both vaccines are administered intramuscularly and should not be given to individuals with FOP.
References
Bergfors E, Hermansson G, Nystrom-Kronander U, Falk L, Valter L, Trollfors B. How common are long- lasting, intensely itching vaccination granulomas and contact allergy to aluminum induced by currently used pediatric vaccines? A prospective cohort study. Eur J Pediatr 173: 1297-1307, 2014
Bergfors E, Trollfors B, Inerot A. Unexpectedly high incidence of persistent itching nodules and delayed hypersensitivity to aluminium in children after the use of adsorbed vaccines from a single manufacturer. Vaccine 22: 64-69, 2003
Carpenter SL, Soucie JM, Presley RJ, Ragni MV, Wicklund BM, Silvey M, Davidson H. Hepatitis B vaccination is effective by subcutaneous route in children with bleeding disorders: a universal data collection database analysis. Haemophilia 21: e39-e43, 2015
Cook IF. Evidence based route of administration of vaccines. Hum Vaccin 26: 67-73, 2008
Hawkridge A, Hatherill M, Little F, Goetz MA, Barker L, Mahomed H, Sadoff J, Hanekom W, Gaiter L. Efficacy of percutaneous versus intradermal BCG in the prevention of tuberculosis in South African infants: randomized trial. BMJ 337:a2052, 2008
Hills SL, Walter EB, Atmar RL, Fischer M. Japanese encephalitis vaccine: recommendations of the Advisory Committee on immunization practices. MMWR 19:68:1-33, 2019
Kimberlin DW, et al. Red Book: 2021-2024 Report of the Committee on Infectious Diseases (32nd Ed). American Academy of Pediatrics. Diphtheria, pp 304-307; Pertussis, pp 578-589; Tetanus, pp 750-755, 2021
Kitterman JA, Kantanie S, Rocke DM, Kaplan FS. Iatrogenic harm caused by diagnostic errors in fibrodysplasia ossificans progressiva. Pediatrics 116: e654-e661, 2005
Lanchoney TF, Cohen RB, Rocke DM, Zasloff MA, Kaplan FS. Permanent heterotopic ossification at the injection site after diphtheria-tetanus-pertussis immunizations in children who have fibrodysplasia ossificans progressiva. J Pediatr 126: 762-764, 1995
Pembroke AC and Marten RH. Unusual cutaneous reactions following diphtheria and tetanus immunization. Clin Exp Dermatol 4: 345-348, 1979
Ragni MV, Lusher JM, Koerper MA, Manco-Johnson M, Krause DS. Safety and immunogenicity of subcutaneous hepatitis A vaccine in children with haemophilia. Haemophilia 6: 98-103, 2000
Ritchey AK. Administration of vaccines to infants and children with hemophilia. A survey of Region III comprehensive hemophilia treatment centers. Blood 106: 4079, 2005
Roush SW, Murphy TV, and the Vaccine-Preventable Disease Table Working Group. Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA 298: 2155-2163, 2007
Schaefer BA, Gruppo RA, Mullins ES, Tarango C: Subcutaneous diphtheria and tetanus vaccines in children with haemophilia: a pilot study and review of the literature. Haemophilia 23: 904-909, 2017
Srivastava A, et al. WFH Guidelines for the Management of Hemophilia, 3rd Ed. Haemophilia 26 Suppl 6: 1-158, 2020
5-8. Immunizations for Influenza in FOP
Influenza (“the flu”) is a major cause of morbidity and mortality worldwide. It is especially dangerous for those with FOP (Scarlett et al., 2004). Every year, flu vaccines are produced based on the predicted strains that will be prevalent in the following cycle. The Centers for Disease Control and Prevention (CDC) recommend flu vaccinations by the end of October in the United States. Other countries may have different schedules and types/brands of vaccines available. Timing of the vaccinations should be determined with the local health care provider.
The most common forms of flu vaccinations are administered intramuscularly (IM) or provided as a live attenuated virus delivered in an intranasal form. In some years, transdermal and intradermal flu vaccines (given via a patch through the skin or injected just under the skin but not into the deeper tissues) may be available. Availability is based on manufacturing and effectiveness assessments that are performed yearly on that batch of vaccine.
Live attenuated flu vaccines are not recommended
The live attenuated intranasal form of the flu vaccine (such as Flumist® in the United States) has been reported to be associated with flares in some patients with FOP (F. Kaplan, Personal communication). This intranasal vaccine is not recommended for patients with FOP or their family members for this reason.
Transdermal or intradermal vaccines are preferred, when available
If transdermal or intradermal forms of the flu vaccine are available, we recommend, as we have for many years, using those routes. Information about the seasonal influenza flu vaccine in the United States can be found on the CDC website: https://www.cdc.gov/flu/vaccines/index.htm
Subcutaneous alternative route for flu vaccine administration
In years when the transdermal or intradermal forms are not available, we recommend that people living with FOP receive the flu vaccine using a modified protocol where the regular flu vaccine is given subcutaneously. Although there is no clear data as to how efficacious this is, prior studies suggest that there will be some efficacy despite being administered by a different route. Note that this will likely require a physician or physician’s office to administer the flu vaccine, as many places (i.e. pharmacies) will not deviate from their normal protocol of intramuscular injection.
For children, have one dose (typically 0.25 ml, depending on the formulation) of the intramuscular vaccine administered subcutaneously. Do not deliver the vaccine intramuscularly.
For adults, either have two doses of the pediatric dose (typically 0.25 ml each) intramuscular vaccine administered subcutaneously, at two different locations. Alternatively, have the regular adult dose (typically 0.5 ml, depending on the formulation) split and administered as two separate subcutaneous injections at two different locations. The locations do not need to be far apart. Injection of 0.5 ml subcutaneously in any location may be uncomfortable. The ICC does NOT recommend to take the vaccine intramuscularly.
Special precautions for vaccination of patients with FOP
For all vaccinations in persons living with FOP, it is recommended that:
An injection site be chosen near a joint or muscle group that has already been affected by HO, and in a location that would not cause complications if heterotopic bone was to form. That way, if a flare does develop, it is less likely to result in loss of mobility.
For all patients, it is recommended to take a dose of acetaminophen or ibuprofen with the vaccine to help with any discomfort that the vaccine may cause.
Vaccinations should not be given within 2 weeks of a flare or flare-like symptoms. It is advised to wait a minimum of 2 weeks but prefer 6-8 weeks because flareups can often occur in temporal clusters. This strategy is used to decrease the chances of a vaccine inducing a subsequent flare.
Family members living in the same home and caretakers should get the standard intramuscular flu vaccination on schedule. The nasal flu spray is NOT recommended for those in close contact with individuals living with FOP because the attenuated virus, though weaker, can still give a mild case of the flu to contacts.
Please keep in mind that those living with FOP should avoid ANY immunizations during an active flare-up – and for at least 6-8 weeks thereafter.
Antivirals – Oseltamivir/Tamiflu
If a person living with FOP or anyone living with or caregiving for a person with FOP develops symptoms suggestive of the flu, they should get prompt evaluation (including evaluation for other infections, such as COVID or RSV) and consider antiviral treatment (i.e. oseltamivir, Tamiflu®). Oseltamivir is only effective against influenza and does not work against the common cold or other viruses. The effectiveness of oseltamivir is highest in the early phase of an infection, so prompt medical attention is important when symptoms start (typically a combination of high fever and upper respiratory symptoms). Nasal swab testing may be needed to confirm infection with influenza. Oseltamivir does not have a long shelf life, so we generally recommend that a prescription be available “on hold” at a 24-hr pharmacy and that the medication be started once influenza infection has been confirmed by a medical care provider (Jefferson et al., 2014).
Infection Prevention for Everyone
Everyone should practice everyday preventive actions to stop the spread of germs as shared by the CDC on their website at https://www.cdc.gov/flu/protect/preventing.htm.
Try to avoid close contact with sick people.
While sick, limit contact with others as much as possible to keep from infecting them.
If you are sick with flu symptoms, CDC recommends that you stay home for at least 24 hours after your fever is gone except to get medical care or for other necessities. (Your fever should be gone for 24 hours without the use of a fever-reducing medicine).
Cover your nose and mouth with a tissue when you cough or sneeze. Throw the tissue in the trash after you use it.
Wash your hands often with soap and water. If soap and water are not available, use an alcohol-based hand rub.
Avoid touching your eyes, nose and mouth. Germs spread this way.
Clean and disinfect surfaces and objects that may be contaminated with germs like the flu.
See Everyday Preventive Actions and Nonpharmaceutical Interventions (NPIs) for more information about actions – apart from getting vaccinated and taking medicine – that people and communities can take to help slow the spread of illnesses like influenza (flu).
References
Jefferson T, Jones MA, Doshi P, Del Mar CB, Hama R, Thompson MJ, Spencer EA, Onakpoya I, Mahtani KR, Nunan D, Howick J, Heneghan CJ. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev 2014 Apr 10;(4)
Scarlett RF, Rocke DM, Kantanie S, Patel JB, Shore EM, Kaplan FS. Influenza-like viral illnesses and flare-ups of fibrodysplasia ossificans progressiva (FOP). Clin Orthop Rel Res 423: 275-279, 2004
5-9. Coronavirus (COVID-19) Precautions & Guidance for FOP Patients & Families
The Coronavirus (COVID-19) pandemic continues to pose a significant risk to the population worldwide with new variants of SARS-CoV-2 virus that are emerging. The ICC recommends that people living with FOP continue to follow precautionary measures to prevent infection from SARS-CoV-2, the virus that causes the COVID-19 illness.
The ICC is providing this update to the prior statement in May 2022. This document focuses on updated information reporting on COVID-19 infection and vaccination in FOP patients, approval on COVID-19 vaccination in children aged 6 months and above, as well as boosters and treatment.
The recommendations are changing rapidly and are country specific. Most countries have ended their emergency regulations for COVID-19.
The ICC does not provide recommendations on whether a patient with FOP should or should not receive a COVID vaccine.
The decision to take a vaccine is a personal one and based on the balance of risks and benefits, and this should be discussed with your medical team. ICC continues to recommend that COVID-19 vaccines be administered that same route that it was approved (i.e., intramuscular).
Additional information about COVID-19 and COVID vaccination in patients with FOP is now published. (https://ojrd.biomedcentral.com/articles/10.1186/s13023-022-02246-4 and https://ojrd.biomedcentral.com/articles/10.1186/s13023-023-02638-0)
Amongst 23 FOP patients who received intramuscular COVID-19 vaccination. Most common symptoms were pain/soreness, tiredness and swelling. These symptoms are similar to those reported by the general population. One out of 23 developed a flare-up. No patients who received the COVID-19 vaccine were hospitalized.
Amongst 19 FOP patients with COVID-19 infection. Most common symptoms were fatigue, loss of taste or smell and cough. Two out 19 FOP patient developed flare ups and 3 patients were hospitalized.
Vaccines are now generally available for children age 6 months or over. ICC does not provide recommendations on whether a patient with FOP should or should not receive a COVID vaccine. Discuss with your medical team, as local recommendations on when to take a vaccine or eligibility for a vaccine may vary.
ICC does not provide recommendations for or against the booster vaccination, but boosters should be considered if you completed vaccinations previously and are in a high risk area. Please consult with your medical team prior to receiving the booster to discuss if a booster is appropriate and safe.
Patients with FOP are at high risk of complications with COVID-19 infection and should discuss with their medical team if use of monoclonal antibodies or anti-retroviral medications would be beneficial, in the event of a SARS-CoV2 infection.
Monoclonal antibodies are given intravenously and are approved for adults and pediatric patients (≥12 years of age weighing ≥40 kg). Those interventions should be started as early as possible and before 10 days of symptoms onset. Note that some monoclonal antibodies are not effective against the newest strains of SARS-CoV2.
Anti-retrovirals are pills that have been approved. These should generally be administered within 5 days of symptoms onset.
Availability and recommendations of the use of these treatments are rapidly changing and country specific. Some of these therapies may not work against strains prevalent in a particular region. Please consult with your local medical team for recommendations
Discuss with your doctors to make sure there are no medication interactions
If you are in a clinical trial, it is important to discuss any vaccines or therapies with your study doctors.
Clinical data about FOP and COVID/SARS-CoV2 infections are published here:
Social and clinical impact of COVID-19 on patients with fibrodysplasia ossificans progressiva | Orphanet Journal of Rare Diseases | Full Text (biomedcentral.com)
https://ojrd.biomedcentral.com/articles/10.1186/s13023-022-02246-4
A follow-up report on the published paper Social and clinical impact of COVID-19 on patients with fibrodysplasia ossificans progressive (biomedcentral.com)
https://ojrd.biomedcentral.com/articles/10.1186/s13023-023-02638-0
Masking continues to be an important component of controlling the spread of SARS-CoV-2. The ICC strongly recommends the use of tight fitting N95, KN95, or KF94 masks whenever possible to protect the wearer from infection by SARS-CoV-2. If these masks are not available or uncomfortable, then wearing a 3-layer surgical mask would be the next best choice.
The ICC is aware of a recent publication suggesting that the use of subcutaneous injection of the vaccine could still induce adequate vaccine response. However, this study likely delivered the vaccine via a shallow intramuscular route. Furthermore, there are multiple reports in the literature of severe reactions to subcutaneous injection of the COVID vaccine. Efficacy of subcutaneous delivery of a COVID vaccine remains unproven. Therefore, the ICC continues to recommend following the manufacturers’ directions for vaccination and NOT taking intramuscular COVID vaccines by the subcutaneous route.
If you decide to take the COVID vaccine or booster, we recommend:
Discuss your plans with your doctor. Review any potential allergies or prior reactions like anaphylaxis that you should consider before taking the vaccine.
If you are in a clinical trial or study, it is important to discuss any vaccines or therapies with your study doctors.
Take the vaccine via the recommended route and dose (i.e., intramuscular (IM) for the currently available vaccines). Safety and efficacy of taking an IM vaccine through the subcutaneous route is not known and could cause a more unexpected inflammatory responses or poor immune reactions and is currently not recommended.
If possible, take the vaccine in a location that is already fused, as the vaccines all appear to induce some local site reaction (arm pain and swelling). For example, if your left hip or right shoulder are fused, you should use the muscle around those sites.
As with other vaccines, patients with FOP should be flare free for at least 2 weeks prior to receiving the vaccine.
Have the injection done by an experienced nurse, physician, or pharmacist.
Use the shortest needle available (this varies with clinical site). The clinician should be aware that patients with FOP may have hidden HO and thinned muscle at the site of the injection. Avoid injecting directly next to existing HO bone if possible.
Prior to the vaccination, have ibuprofen or acetaminophen available. Also, have a course of prednisone for flares available.
The symptoms reported by patients with FOP after a COVID vaccination are similar to those reported for the general population (low grade fever, headache, muscle aches, fatigue, etc.).
Make sure your physician is familiar with the ICC Treatment guidelines, specifically on vaccinations and flare management (see below). Notify your physician you plan to do the vaccine, and when.
On the day of the injection:
Your local team may not allow you to take ibuprofen or acetaminophen prior to the injection (this is because they may screen for COVID symptoms first).
After you receive your injection, there may be a brief observation period.
After that is completed, take ibuprofen (2 to 3 times/day) or acetaminophen (2-3 times/day) following the label instructions, for the next 48 hrs, regardless of your symptoms.
Rest and stay hydrated.
In the event of a flare, contact your physician for guidance. You may need to do a short course of prednisone, but this needs to be balanced with the immunosuppressive effects of steroids. The usual flare dosing is prednisone 2 mg/kg/day up to 100 mg, for 4 days; your physician may recommend starting at a lower dose, depending on your symptoms.
Even if you take the vaccine, you still need to continue physical distancing, wearing masks, and appropriate hand washing
The ICC can’t guarantee that these steps will “work” to prevent complications. Flare or flare-like activity has been reported in patients with FOP who receive a COVID vaccine. All medications and treatments have risk, so it is important to discuss your specific situation with your doctor as you decide whether the vaccine is appropriate for your situation.
Make sure that you complete the full immunization regimen recommended (i.e., do both doses if the vaccine recommends 2 doses)
Discuss with your physician if you should do a booster and if that is appropriate for you, such as to cover local SARS-CoV2 variants. This is an area of active investigation so will need to be updated as the ICC receives more information.
How does the development of a vaccine change things?
The development and distribution of vaccines have had a major impact on the COVID pandemic.
Vaccine outcomes and the emergence of variants are areas of intense ongoing study worldwide, and the field continues to rapidly evolve.
The duration of immunity conferred by the vaccines is unknown but does not seem to be lifelong.
The ICC recommends that FOP family members and caregivers be fully vaccinated for SARS-CoV2 if safely available for them.
Vaccinations can take 2+ weeks to show any efficacy, so there is no protection immediately after vaccination. In addition, vaccines do not confer absolute immunity to the SARS-CoV-2 virus, and may not have activity against all forms of the SARS-CoV-2 virus.
Anyone who receives a vaccine should still continue with masking, hand hygiene, and physical distancing.
Please discuss with your local care providers regarding benefits and risk of any locally approved vaccines and boosters.
It’s very important to maintain social distancing and wearing a mask when around members outside your household
Recommendations if a patient with FOP or caregiver tests positive for SARS-CoV2
Notify your primary care physician to help coordinate care.
Follow your local guidelines for isolation/quarantine and the needed durations and procedures. Everyone, including the person with the positive SARS-CoV2, should wear a mask at all times to avoid transmission.
Patients who are negative for SARS-CoV2 but have similar symptoms should be tested for influenza.
Patients with FOP are at high risk of complications with COVID-19 infection and should discuss with their medical team if use of monoclonal antibodies or anti-retroviral medications would be beneficial, in the event of a SARS-CoV-2 infection. The main reason for treatment would be to reduce respiratory complications, as patients with FOP are at high risk of breathing complications and are difficult to intubate. However, access to these medications may be limited in your area. Please discuss with your physician if these medications are an option and appropriate for you.
Monoclonal antibodies are given intravenously and are approved for adults and pediatric patients (≥12 years of age weighing ≥40 kg). Those interventions should be started as early as possible and before 10 days of symptoms onset.
Anti-retrovirals are pills that have been approved for treatment of COVID-19. These should generally be administered within 5 days of symptoms onset.
Availability and recommendations of the use of these treatments are rapidly changing and country specific. Please consult with your local medical team.
Discuss with your physician about any potential medication interactions prior to starting anti-viral therapies
References
Kou S, Kile S, Kambampati SS, Brady EC, Wallace H, De Sousa CM, Cheung K, Dickey L, Wentworth KL, Hsiao EC. Social and clinical impact of COVID-19 on patients with fibrodysplasia ossificans progressiva. Orphanet J Rare Dis 2022 Mar 4;17(1):107
Wallace H, Lee RH, Hsiao EC. A follow-up report on the published paper Social and clinical impact of COVID-19 on patients with fibrodysplasia ossificans progressiva. Orphanet J Rare Dis 2023 Mar 20;18(1):61
5-10. Acute & Chronic Pain Management in FOP
General Considerations
According to the IFOPA Patient Registry (Mantick et al., 2018), almost 90% of individuals with FOP have pain complaints. Major causes of acute pain in FOP are musculoskeletal in origin and include flare-ups, transient bursitis, inflammation of osteochondromas, muscle fatigue, and fracture through heterotopic bone.
The most important aspect of acute pain management is distinguishing acute pain due to flare-ups versus other etiologies. In a study on the natural history of flare-ups in FOP (Pignolo et al, 2016), the most painful flare-ups occur in the hips and knees. Quantitative sensory testing of patients with FOP revealed significant heat and mechanical pain hypersensitivity, suggesting a novel neuropathic pain phenotype (Yu et al., 2023).
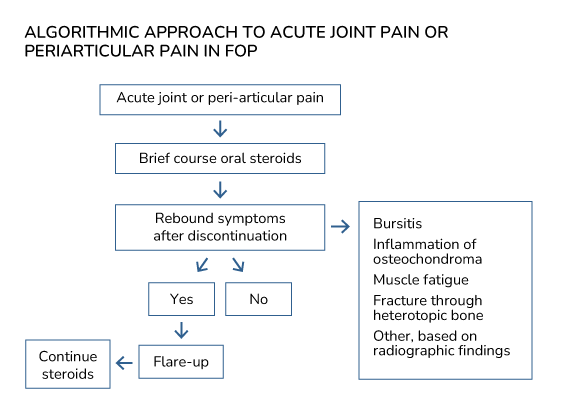
Based on a recent evaluation of clinical and radiographic findings of acute hip pain in FOP (Kaplan et al., 2018; see section on Differential diagnosis of hip pain), an algorithmic approach to acute joint or periarticular pain may be proposed (Figure). After an initial brief course of steroids to empirically treat for a possible flare-up, observation for rebound symptoms with discontinuation is the critical node for further treatment decisions. The absence of rebound symptoms (i.e., resolution of pain complaints) suggests that the etiology for pain is not related to a flare-up. The presence of continued or worsening symptoms after discontinuation, however, strongly suggests a flare-up as the likely cause. Plain radiographs of the involved joint can be helpful in the management of acute periarticular pain.
Common causes of chronic pain in FOP include neuropathies, arthritis, generalized chronic pain syndrome in advanced FOP, and other causes of pain such as gastrointestinal pain (see section on gastrointestinal issues). The approach to chronic pain relies on distinguishing between neuropathic and nociceptive etiologies. Neuropathic pain results from damage to or pathology within the nervous system and can be central or peripheral. In FOP, neuropathies are the major cause of neuropathic pain, related to entrapment syndromes and/or nerve damage, and may be caused by peripheral or central nervous system phenomena intrinsically related to the cause of FOP (Kan et al., 2011; Kan et al., 2012; Peng et al., 2021; Yu et al, 2023). Nociceptive pain is caused by stimuli that threaten or provoke actual tissue damage. In FOP, the major causes of nociceptive pain are musculoskeletal pain (e.g., back pain, myofascial pain syndrome, ankle pain), inflammatory pain, and pain due to mechanical/compressive causes (e.g., visceral pain from expanding HO).
General principles of pain management should be applied to the treatment of chronic pain in FOP. The best approach is to target the etiology of the pain, whenever known, and to make reasonable attempts at understanding at least the type of pain when the etiology is unknown. Optimal outcomes often result from multiple approaches utilized in concert, coordinated via a multidisciplinary team. Some adjuvant, non- pharmacologic modalities can be effective in pain management. Finally, treatment of depression may provide pain relief separate from correction of the mood disorder.
Treatment of chronic pain is based on neuropathic versus nociceptive components. There is consensus among guidelines on pain management of non-FOP pain syndromes that first-line agents for neuropathic pain include either calcium channel alpha 2-delta ligands (e.g., gabapentin or pregabalin) or tricyclic antidepressants (Gilron et al., 2015; Finnerup et al., 2015). Serotonin norepinephrine uptake inhibitors (SNRIs) are identified as either first or second-line agents (e.g., duloxetine, venlafaxine), although they may be preferred to tricyclic antidepressants. Among the tricyclic antidepressants, side effect profiles appear to favor the secondary amine tricyclics (e.g., nortriptyline and desipramine), although the efficacy is the same for other tricyclics such as amitriptyline. Combination therapy is often required, because less than half of patients with neuropathic pain will respond to a single agent. However, evidence is scant regarding the efficacy and safety of combination treatment. Other second-line agents that may be used include tramadol and other antiepileptics (e.g., carbamazepine or oxcarbazepine). Opioids should be considered a third-line option in FOP, both because of abuse potential and the fact that activation of mast cells and the systemic release of histamine are common side effects of opioids, especially codeine and meperidine (Blunk et al., 2004). Topical agents can be used as adjunctive therapy as appropriate.
The pharmacologic approach to nociceptive pain first begins with an evaluation of risk factors that may contraindicate, limit, or otherwise call attention to the possibility of potential side effects related to the use of non-steroidal anti-inflammatory agents (NSAIDS), the mainstay of treatment (McCormack, 1994; Roelofs et al., 2008). Risk factors include advanced age, renal, hepatic, cardiovascular disease or risk, peptic ulcer, and glucocorticoid use. The latter is directly applicable to FOP, and systemic NSAIDS should not be used concomitantly with steroids. After risk factor evaluation, the next step to nociceptive pain management is an assessment of pain level. Mild to moderate pain can initially be treated with topical agents (see section on Topical Agents) and/or acetaminophen/paracetamol.
Pain not controlled by topical agents or acetaminophen/paracetamol should be managed with NSAIDs plus a proton-pump inhibitor or COX-2 inhibitor with or without acetaminophen/paracetamol. There is controversy over the maximal safe daily dose of acetaminophen, but a 3g to 3.25g daily dose appears to be within a safe maximal range (Heard et al., 2007).
Treatment of moderately severe to severe pain without an inflammatory component or with risk factors for NSAID use should begin with acetaminophen/paracetamol and advanced to tricyclic antidepressants if pain is not adequately controlled. Moderately severe to severe pain with an inflammatory component should be initially treated with NSAIDs plus a proton-pump inhibitor or COX-2 inhibitor with or without acetaminophen/paracetamol. Use of COX-2 inhibitors may decrease the likelihood of gastrointestinal toxicity from NSAIDs (Silverstein et al., 2000). Tricyclic antidepressants can also be used if pain is not adequately controlled. The addition of baclofen or other muscle relaxant may be appropriate for a short period of time if there is a spasmodic component to the pain (see section on Muscle Relaxants). For the reasons stated above, opioids should also be considered a third-line option in FOP for the management of nociceptive pain.
There are many causes of acute and chronic pain in FOP, and each individual must be carefully evaluated before effective treatment can be planned and implemented (Kaplan et al., 2008). Many FOP flare-ups, especially those around the hips and knees, are extremely painful and may require a brief course of well-monitored narcotic analgesia in addition to the use of non-steroidal anti-inflammatory medications, COX-2 inhibitors, and/or oral or IV glucocorticoids. Other types of transient pain syndromes may be caused by neuropathies resulting from acute flare-ups, transient bursitis, inflammation of osteochondromas, arthritis and muscle fatigue, to mention only a few.
To date, much remains unknown regarding the dynamics of pain and emotional health in FOP during flare- up and also quiescent, non-flare-up disease phases. In order to elucidate the occurrence and effect of pain in FOP, a study analyzed patient-reported outcomes measurement information system-based questionnaires completed by 99 patients participating in the international FOP Registry over a 30-month period (Peng et al., 2019). The study observed that while moderate to severe pain (≥4, 0-10 pain scale) was commonly associated with flare-ups (56-67%), surprisingly, 30-55% of patients experienced similar pain levels during non-flare-up states. Furthermore, independent of the flare-up status, the severity of pain in FOP patients was found to be inversely correlated with emotional health, physical health and overall quality-of-life. These findings strongly suggest the need for an improved understanding of pain and emotional health in FOP during flare-up and quiescent periods (Peng et al., 2019).
Those with chronic pain of diffuse musculoskeletal origin may require more specialized pain management programs directed by pain management specialists. Attempts should be made to minimize chronic discomfort and maximize physical and cognitive function. In most cases, narcotic agents should be avoided to minimize the risk of dependency on these agents. While some may require chronic narcotic analgesics late in the course of their disease process, attempts should be made to monitor this carefully to avoid constipation and respiratory suppression.
Use of alternative of complementary therapies have not been well investigated but may provide options for reducing systemic pain medication use. Pain neuroscience education may be useful and has been shown to be an effective intervention in all types of pain (Louw et al., 2016). Acupuncture is not recommended due to the potential for tissue trauma.
Other complementary or integrative medicine techniques for pain management should be considered, including the use of biofeedback, therapy pools, therapeutic relaxation, auto-hypnosis, cognitive behavioral therapy, gentle massage/acupressure, and medicinal marijuana (where available). These therapies should be discussed with the treating physician to ensure that there are no adverse interactions with other medications or therapies, or contraindications for an individual patient. Also, procedures such as acupressure and gentle massage need to be performed in a way that does not increase the risks of trauma or inducing a flare. Please discuss with the primary care physician or local pain management team for referrals to a reputable complementary or integrative medicine practitioner.
For those with more chronic pain management issues, a consultation with a pain management specialist may be helpful and is recommended.
Medical Cannabis
Anecdotal use of medical cannabis among patients with FOP is known to occur. This class of drugs is represented by a group of compounds derived from the subspecies of the plant genus Cannabis and delivered for potential beneficial effects through inhalation, swallowing, and topical application to skin or buccal mucosa. At the time of this writing, 33 U.S. states and the District of Columbia have programs authorizing cannabis use for specific medical conditions, with varying regulations in other countries. Most states allow its use for a variety of conditions, with those most related to FOP including cachexia/wasting syndrome, muscle spasticity, severe and chronic pain, and severe nausea (Belendiuk et al., 2015). In the U.S., clinicians do not prescribe medical cannabis but only certify for the condition that qualifies patients under state programs.
The National Academies of Sciences, Engineering, and Medicine (NASEM) conducted a comprehensive review on the health effects of cannabis and cannabinoids (NASEM, 2017). The NASEM reported conclusive or substantial evidence that cannabis or cannabinoids are effective for chronic pain in adults, as anti-emetics, and for improvement of muscle spasticity. Chronic pain is the most common reason individuals use medical cannabis; however, its use needs to be part of a comprehensive symptom management strategy rather than a single modality for pain control, underscoring the importance of continuing other therapies. In this context, it is noteworthy that use of medical cannabis has been associated with significantly lower opioid overdose mortality rates (Bachhuber et al., 2014).
Oral cannabis extracts from naturally occurring phytocannabinoids, orally administered Δ9- Tetrahydrocannabinol (THC) and nabiximols (combination THC-cannabidiol [CBD]) are the usually administered forms, but synthetic cannabinoids are also available. Among the different preparations, evidence-based claims regarding clinical efficacy can only be made for smoked or vaporized plant flower, plant-derived oral THC, THC-CBD combinations, and synthetic THC. However, the NASEM noted that there is substantial evidence for an association between cannabis smoking and respiratory disease, motor vehicle collisions (MVCs), lower birth weight of offspring, and schizophrenia or other psychoses (NASEM, 2017). Caution should be exercised when considering medical cannabis for patients under the age of 25 years because brain development continues until this age, and the potential lasting impact of cannabis on cognitive performance is unknown (NASEM, 2017). Also, it is difficult to extrapolate available evidence on medical cannabis given the differences in products obtained from state-run programs due to horticultural techniques, drug extraction methods, and drug delivery modalities.
References
Bachhuber MA, Saloner B, Cunningham CO, Barry CL. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999-2010 [published correction appears in JAMA Intern Med. 2014;174(11):1875]. JAMA Intern Med 174: 1668-1673, 2014
Belendiuk KA, Baldini LL, Bonn-Miller MO. Narrative review of the safety and efficacy of marijuana for the treatment of commonly state-approved medical and psychiatric disorders. Addict Sci Clin Pract 2015; 10:10
Blunk JA, Schmelz M, Zeck S, Skov P, Likar R, Koppert W. Opioid-induced mast cell activation and vascular responses is not mediated by mu-opioid receptors: an in vivo microdialysis study in human skin. Anesth Analg 98: 364-370, 2004
Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 14: 162, 2015
Gilron I, Baron R, Jensen T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc 90: 532, 2015
Heard K, Green JL, Bailey JE, et al. A randomized trial to determine the change in alanine aminotransferase during 10 days of paracetamol (acetaminophen) administration in subjects who consume moderate amounts of alcohol. Aliment Pharmacol Ther 26: 283, 2007
Kan L, Kitterman JA, Procissi D, Chakkalakal S, Peng CY, McGuire TL, Goldsby RE, Pignolo RJ, Shore EM, Kaplan FS, Kessler JA. CNS demyelination in fibrodysplasia ossificans progressiva. J Neurol 259: 2644-2655, 2012
Kan L, Lounev VY, Pignolo RJ, Duan L, Liu Y, Stock SR, McGuire TL, Lu B, Gerard NP, Shore EM, Kaplan FS, Kessler JA. Substance P signaling mediates BMP-dependent heterotopic ossification. J Cell Biochem 112: 2759-2772, 2011
Kaplan FS, Al Mukaddam M, Pignolo RJ. Acute unilateral hip pain in fibrodysplasia ossificans progressiva (FOP). Bone 109: 115-119, 2018
Kaplan FS, Le Merrer M, Glaser DL, Pignolo RJ, Goldsby R, Kitterman JA, Groppe J, Shore EM. Fibrodysplasia ossificans progressiva (FOP). Best Practice & Research – Clinical Rheumatology 22: 191-205, 2008
Louw A, Zimney K, Puentedura EJ, Diener I. The efficacy of pain neuroscience education on musculoskeletal pain: A systematic review of the literature. Physiother Theory Pract 32: 332-355, 2016
Mantick N, Bachman E, Baujat G, Brown M, Collins O, De Cunto C, Delai P, Eekhoff M, Zum Felde R, Grogan DR, Haga N, Hsiao E, Kantanie S, Kaplan F, Keen R, Milosevic J, Morhart R, Pignolo R, Qian X, di Rocco M, Scott C, Sherman A, Wallace M, Williams N, Zhang K, Bogard B. The FOP Connection Registry: Design of an international patient-sponsored registry for Fibrodysplasia Ossificans Progressiva. Bone 109: 285-290, 2018
McCormack K. Non-steroidal anti-inflammatory drugs and spinal nociceptive processing. Pain 59: 9, 1994
National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press, 2017
Peng K, Cheung K, Lee A, Sieberg C, Borsook D, Upadhyay J. Longitudinal evaluation of pain, flare-Up, and emotional health in fibrodysplasia ossificans progressiva: Analyses of the international FOP registry. JBMR Plus 2019 Mar 1;3(8):e10181.
Peng K, Karunakaran KD, Labadie R, Veliu M, Cheung C, Lee A, Yu PB, Upadhyay J. Suppressed prefrontal cortex oscillations associate with clinical pain in fibrodysplasia ossificans progressiva. Orphanet J Rare Dis 2021 Jan 30;16(1):54.
Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine 33:1766, 2008
Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti- inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 284: 1247, 2000
Yu X, Ton AN, Niu Z, Morales BM, Chen J, Braz J, Lai MH, Barruet E, Liu H, Cheung K, Ali S, Chan T, Bigay K, Ho J, Nikolli I, Hansberry S, Wentworth K, Kriegstein A, Basbaum A, Hsiao EC. ACVR1- activating mutation causes neuropathic pain and sensory neuron hyperexcitability in humans. Pain 164: 43- 58, 2023.
5-11. Neurological Issues in FOP
Neurological symptoms are commonly reported by patients with FOP. To determine the prevalence of neurological symptoms and their characteristics in individuals with FOP, a worldwide survey was conducted of the 470 patient members of the International FOP Association (IFOPA) using a questionnaire about neurological symptoms. There were 168 responses (105 females, 63 males; age 1.5-68 years) from 30 countries representing 36% of IFOPA members.
Chronic neurological symptoms were reported by 86 (51%). Prevalence of neuropathic pain (NP) was significantly increased (p < 0.001) compared to the general population, and tenfold more common in females (15%) than males (1.6 %). Of those with NP, 94 % reported other sensory abnormalities.
Prevalence of recurrent severe headaches (HA) (26 %) was similar to that in the general population, but prevalence in females with FOP (36%) was almost fourfold greater than in males. Prevalence of NP, HA, and other sensory abnormalities was substantially higher in post-pubertal females; 33 % reported symptoms worsened during menstrual periods. Worsening of neurological symptoms during FOP flare-ups was reported by 23%. Three patients with FOP (1.8%) reported myoclonus, prevalence much greater than reported in the general population (p < 0.001). This worldwide survey indicated that neurological symptoms are common in FOP. These symptoms are plausibly related to dysregulated BMP signaling in the central and/or peripheral nervous systems (Kitterman et al., 2012). Similar findings were present in a study involving patients with FOP who were enrolled in the IFOPA Registry (Yu, et al., 2023).
To further elucidate these atypical neurologic symptoms, two mouse models of dysregulated BMP signaling relevant to FOP were evaluated for potential CNS pathology through non-invasive magnetic resonance imaging (MRI) studies and histological and immunohistochemical approaches. In one model, BMP4 was over-expressed under the control of the neuron-specific enolase promoter; the second model is a knock-in of the classic FOP mutation in ACVR1. MRI scans of four FOP patients were retrospectively examined.
Demyelinated lesions and focal inflammatory changes of the CNS in both mouse models were consistently observed but not in wild-type controls. CNS white matter lesions were also found in each of the four FOP patients examined.
These findings suggest that dysregulated BMP signaling disturbs normal homeostasis of target tissues, including CNS where focal demyelination may manifest as the neurologic symptoms frequently observed in FOP (Kan et al., 2012). While most CNS lesions in FOP patients are incidental, asymptomatic and well- compensated, consistent developmental findings in the CNS in some of the severe FOP variants support the hypothesis that over-activity of BMP signaling may have consequences for development and repair of the CNS (Kaplan et al., 2015; Severino et al., 2016).
In addition, studies have shown that the ACVR1 gene is important in nociceptors (cells that sensing pain and irritant signals). Human induced pluripotent stem cells (iPSCs) carrying the ACVR1R206H mutation showed increased activity in response to irritant stimuli, supporting the clinical finding of nociceptive pain in patients with FOP.
Practically and in summary, patients with FOP have reported a higher incidence of neurological concerns including pain both during and outside of an FOP flare-up. The sources of pain should be carefully explored. Patients having neuropathic pain may benefit from certain classes of medications, such as gabapentin and pregabalin – the appropriateness and choice of medications should be discussed with the patient’s physicians. In addition, patients with FOP who experience chronic pain should consider having a clinical pain management team for evaluation and treatment. Finally, some individuals with FOP report chronic headaches as noted above. If headaches persist, patients should be referred to a neurologist, who can make recommendations for detailed evaluation and treatment.
References
Kan L, Kitterman JA, Procissi D, Chakkalakal S, Peng C-Y, McGuire TL, Goldsby RE, Pignolo RJ, Shore EM, Kaplan FS, Kessler JA. CNS demyelination in fibrodysplasia ossificans progressiva. J Neurol 259: 2644-2655, 2012
Kaplan FS, Kobori JA, Orellana C, Calvo I, Rosello M, Martinez F, Lopez B, Xu M, Pignolo RJ, Shore EM, Groppe JC. Multi-system involvement in a severe variant of fibrodysplasia ossificans progressiva (ACVR1c.772G>A; R258G): a report of two patients. Am J Med Genetic A 167A: 2265-2271, 2015
Kitterman JA, Strober JB, Kan L, Rocke DM, Cali A, Peeper J, Snow J, Delai PLR, Morhart R, Pignolo RJ, Shore EM, Kaplan FS. Neurological symptoms in individuals with fibrodysplasia ossificans progressiva. J Neurol 259: 2636-2643, 2012
Severino M, Bertamino M, Tortora D, Morana G, Uccella S, Bocciardi R, Ravazzolo R, Rossi A, Di Rocco
M. Novel asymptomatic CNS findings in patients with ACVR1/ALK2 mutations causing fibrodysplasia ossificans progressiva. J Med Genet 53: 859-864, 2016
Yu X, Ton AN, Niu Z, Morales BM, Chen J, Braz J, Lai MH, Barruet E, Liu H, Cheung K, Ali S, Chan T, Bigay K, Ho J, Nikolli I, Hansberry S, Wentworth K, Kriegstein A, Basbaum A, Hsiao EC. ACVR1- activating mutation causes neuropathic pain and sensory neuron hyperexcitability in humans. Pain 164: 43- 58, 2023
5-12. Developmental Arthropathy and Degenerative Joint Disease in FOP
FOP is a disease of not only progressive heterotopic ossification, but also widespread and extensive developmental arthropathy and associated degenerative joint disease.
In a study by Towler and colleagues (Towler et al, 2020), widespread evidence of developmental arthropathy was found throughout the axial and appendicular skeleton in all age groups. Asymmetric narrowing and subchondral sclerosis, radiographic hallmarks of degenerative arthritis, were present throughout the joints of the normotopic skeleton.
The hips of FOP patients were frequently malformed and dysplastic. Osteophytes (bone spurs) were common in the hips and knees of individuals who have FOP in all age groups. The costovertebral joints, intervertebral facet joints throughout the spine, and proximal tibio-fibular joints frequently showed partial or total intra-articular ankylosis (fusion), particularly after age 13. Degenerative joint phenotypes after age 13, were found particularly in the spine, sacroiliac joints, and lower limbs.
Thus, associated degenerative joint disease occurs at multiple sites, starts in adolescence and progresses throughout life. These phenotypes appear to be uncoupled from heterotopic bone formation, indicating a potential role for ACVR1 in the development and progression of degenerative joint disease. Thus, the effects of ACVR1 mutation on the normotopic skeletons of individuals who have FOP extend beyond malformation of the great toes and include both morphological defects and developmental arthropathy. These findings have extraordinary relevance for understanding the natural history of FOP, for treating the arthritis associated with FOP throughout life (especially in adolescence and adulthood) and for designing and evaluating clinical trials with emerging therapeutics.
References
Towler OW, Shore EM, Kaplan FS. Skeletal malformations and developmental arthropathy in individuals who have fibrodysplasia ossificans progressiva. Bone 130: 115116, 2020
5-13. Differential Diagnosis of Hip Pain in FOP
Flare-ups of the hips are among the most feared and disabling complications of FOP. In a recent study (Kaplan et al, 2018), 25 consecutive individuals with classic FOP who presented with acute unilateral hip pain were evaluated. All 25 individuals were suspected of having a flare-up of the hip based on clinical history and a favorable response to a four-day course of high-dose oral prednisone. Ten individuals (40%) experienced rebound symptoms of pain and/or stiffness within seven days after discontinuation of prednisone and all ten subsequently developed HO or decreased mobility of the affected hip. None of the 14 individuals who experienced sustained relief of symptoms following a course of oral prednisone experienced HO or decreased mobility.
Incidental radiographic findings at the time of presentation were multifactorial and included osteochondromas of the proximal femur (18/25; 72%), degenerative arthritis (17/25; 68%), developmental hip dysplasia (15/25; 60%), previously existing HO (12/25; 48%), intra-articular synovial osteochondromatosis (8/25; 32%) or traumatic fractures through pre-existing heterotopic bone (1/25; 4%). Developmental joint pathology may confound clinical evaluation of hip pain in FOP.
The most useful modality for suspecting an ossification-prone flare-up of the hip was lack of sustained response to a brief course of oral prednisone. Evaluation of soft tissue edema by ultrasound or magnetic resonance imaging showed promise in identifying ossification-prone flare-ups and warrants further analysis in prospective studies; however, plain radiographs were able to reliably identify the likely causes of hip pain unrelated to flare-up. Interestingly, most fractures treated nonoperatively in individuals with FOP heal with few flareups, impaired mobility, or heterotopic ossification (Lindborg et al., 2023).
References
Kaplan FS, Al Mukaddam M, Pignolo RJ. Acute unilateral hip pain in fibrodysplasia ossificans progressiva (FOP). Bone 109: 115-119, 2018
Lindborg CM, Al Mukaddam M, Baujat G, Cho TJ, De Cunto CL, Delai PLR, Eekhoff EMW, Haga N, Hsiao EC, Morhart R, de Ruiter R, Scott C, Seemann P, Szczepanek M, Tabarkiewicz J, Pignolo RJ, Kaplan FS. Most Fractures Treated Nonoperatively in Individuals With Fibrodysplasia Ossificans Progressiva Heal With a Paucity of Flareups, Heterotopic Ossification, and Loss of Mobility. Clin Orthop Relat Res 481: 2447-2458, 2023
5-14. Limb Swelling in FOP
Limb swelling is a common problem in patients who have FOP, yet little is known about this complication. In a published study, detailed medical records were reviewed on a large group of FOP patients to determine the prevalence and natural history of limb swelling (Moriatis et al., 1997). Acute swelling of the limbs occurred in association with flare-ups of the condition in nearly all cases. Acute swelling in the upper limbs was focal and nodular in contrast to acute swelling in the lower limbs, which was more diffuse. The intense angiogenesis and edema seen on histopathologic evaluation of preosseous FOP lesions may play a role in the pathogenesis of the limb swelling. In addition, proximal lesions in the limb may cause a mechanical blockage of distal limb lymphatic drainage thus exacerbating the swelling.
The acute and often severe limb swelling seen with acute flare-ups of FOP is ascribed to the intense inflammation, angiogenesis and capillary leakage demonstrable in the early FOP lesions. Limb swelling associated with an acute FOP flare-up may grow to extraordinary and alarming size and lead to extravascular compression of nerves and tissue lymphatics. The appearance of such massive acute swelling in the lower limbs can provoke serious considerations of a deep vein thrombosis.
Massive tissue edema may last for 3-6 months after the onset of acute swelling. As fibrocartilaginous tissue matures into chondro-osseous tissue and finally into bone, swelling diminishes. During the following six months, swelling may regress slowly or may persist as chronic limb swelling. As skeletal muscle in the lower limbs is replaced by heterotopic bone, the normal pumping action of the muscle is lost, further exacerbating lymphatic stasis and dependent edema. Progressive ankylosis of the joints continues inexorably and loss of mobility ensues, further increasing venous and lymphatic stasis and dependent edema (Moriatis et al., 1997).
When lymphedema occurs, it is critical to avoid infections and the vicious cycle of lymphedema-cellulitis- lymphedema. Streptococcal cellulitis associated with lymphedema can be aggressive with severe symptoms and morbidity. Prophylactic skin care of the affected limb in preventing ulcers, dermatitis, macerations and tinea pedis is also important in reducing the portal of entry for micro-organisms. Episodes of cellulitis can damage the lymphatic system and predispose to recurrent episodes of cellulitis (Al Niaimi & Cox, 2009).
Some patients who have advanced FOP involving the lower limbs have venous stasis in addition to lymphedema. Definitive studies to exclude deep vein thrombosis may be difficult to obtain and interpret due to severe existing deformity and joint ankylosis from previous flare-ups. A decision to anticoagulate a patient should not be made without substantial evidence of deep vein thrombosis. The differential diagnosis of acute upper limb swelling is not nearly as difficult as is the differential diagnosis of acute lower limb swelling in patients who have FOP. Differences in the regional appearance of the FOP lesions cannot be explained at the present time but may be due to mechanical factors affecting aponeuroses and fascial planes (Moriatis et al., 1997).
Limb swelling is often difficult to treat effectively in patients who have FOP. Non-steroidal anti- inflammatory medications and glucocorticoids generally have not been effective. Support stockings are poorly tolerated by most patients, and elevation of the affected limbs is often impossible because of ankylosis of the major joints, especially later in the disease process. When tolerated, support stockings may be helpful. The use of pneumatic compression devices has not been evaluated. Additionally, many have reported anecdotal beneficial effects following treatments at lymphedema clinics.
There are two different types of lymphedema therapy. The Vodder method is most often used in clinics, though the Chikly method, which involves “mapping” the lymph flow and potentially using alternate pathways to reroute the lymph flow, may be more useful for FOP patients for whom HO and/or on-going flare-ups may interfere with the normal channels of lymph flow (Chikly et al., 2000; Chikly et al., 2005). Lymphedema clinics can also provide patients with resources to help control lymphedema and are highly recommended.
References
Al Niaimi F, Cox N. Cellulitis and lymphoedema: A vicious cycle. J Lymphoedema 4: 38-42, 2009
Chikly, BJ. Manual techniques addressing the lymphatic system: origins and development. J Am Osteopathic Association 105: 457-464, 2005
Chikly, BJ. Lymph Drainage Therapy (LDT) Manual Lymphatic Mapping and its Clinical Applications to Lymphedema. The National Lymphedema Network (NLN) Publication 12(3), 2000
Moriatis JM, Gannon FH, Shore EM, Bilker W, Zasloff M, Kaplan FS. Limb swelling in patients who have fibrodysplasia ossificans progressiva. Clin Orthop Rel Res 336: 247-253, 1997
5-15. Pressure Ulcers in FOP
Skin breakdown and pressure sores are common and troublesome problems in individuals who have advanced FOP. Skin breakdown can occur from increased pressure over normotopic or heterotopic bone.
Pressure ulcers can develop suddenly, progress rapidly, and be difficult to treat (Reddy et al., 2001; Boyko et al., 2018). Preventive measures include:
Adequate nutrition
Daily skin inspections
Frequent changes in position
Use of a pressure-reducing mattress or bed
Use of pressure-reducing dressings or cushions. If a pressure sore is detected at an early stage when the skin is erythematous but there is no open sore, it will be much easier to treat. Pressure sores involving open wounds require considerably more care.
Follow these suggestions as soon as a problem is identified:
Change positions frequently and use special cushions designed to relieve pressure.
Keep the area clean to prevent infection. A stage I wound (no open skin) can be gently washed with water and mild soap. Anything more serious should be washed with saline (salt) solution, which can be obtained from a pharmacy. Avoid using antiseptics such as hydrogen peroxide or iodine which can damage the skin and delay healing.
Pay special attention to the folded areas of the skin and keep them free of moisture. These areas may be especially problematic for FOP patients when little air can circulate. Keeping these areas dry is very important and simple measures like the use of a thin microfiber washcloth can be helpful.
Use a special dressing/bandage that protects wounds and helps promote healing. Name brands include Tegaderm and Duoderm. These dressings help keep the wound moist (to promote cell growth) while keeping the surrounding tissue dry. They should not be used on lesions that might be infected.
Whirlpool baths can be helpful because they help keep the skin clean and naturally remove dead tissue.
If necessary, damaged tissue can be removed. A wound needs to be free of dead and/or infected tissue to heal properly. There are several ways that this can be done safely, even in FOP.
Perforating bone may be removed under corticosteroid coverage when absolutely necessary.
Coordination with a wound care team is important.
References
Boyko TV, Longaker MT, Yang GP. Review of the Current Management of Pressure Ulcers. Adv Wound Care 7: 57–67, 2018
Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systemic review. JAMA 296: 974-984, 2006
5-16. Dermatology & FOP
The skin is the largest organ of the human body, and often reflects systemic diseases and their consequences, providing clues to diagnosis. Over many years, doctors and researchers have noticed a connection between FOP and some skin, hair, and nail conditions. Although many aspects of these anecdotal dermatologic findings will be the subjects of future studies, it is possible to state that these conditions can be roughly divided in 4 main types:
Dermatologic conditions related to a consequence of FOP – immobility, position of the body, poor blood circulation.
Dermatologic conditions related to the use of drugs (prednisone, NSAIDs, analgesics, and drugs involved in clinical trials).
Dermatologic conditions related to FOP itself – linked to the genetic mutation and signaling pathway.
Dermatologic conditions unique to each individual with FOP – genetic, environmental, and/or occupational.
Dermatologic conditions related to a consequence of FOP
Pressure ulcers are a common skin problem that is often seen in individuals with FOP. Heterotopic bone can occlude the blood supply to the skin and create an ulcer. It is important to avoid this problem by cushioning vulnerable bony prominences and treating pressure ulcers as soon as they are found.
Heterotopic bone often crosses joints, creating body folds that are exceedingly difficult to reach and clean, creating fertile fields for the development of intertriginous lesions, as well as fungal and bacterial infections. Health care professionals must be aware of these possible conditions and minimize the risk of serious infections that can lead to sepsis (Kang et al., 2019).
Heterotopic bone can also lead to compromised circulation as the compression of blood and lymphatic vessels creates poorly oxygenated skin that becomes dry and eczematous. Avoiding hot showers, hot temperatures, and soaps containing lauryl sulfates is recommended. Also, skin moisturizers are recommended. A dermatologist should be consulted about the possible use of steroids and antibiotics (Kang et al., 2019).
Some patients with advanced FOP have reported blisters in the absence of friction or trauma. This may occur from compromised lymphatic circulation. If lymphatic pressure is elevated, tense blisters containing transparent lymphatic fluid may form.
The compression of lymphatic vessels can also be the cause of lymphedema and in extreme cases elephantiasis of the limb with ulcers and verrucous lesions. An ultrasound examination can help to rule out deep venous thrombosis (Moriatis et al., 1997).
Dermatologic conditions related to the use of medications
Skin, nails, and hair can be affected by the use of certain medications. Many medications can be implicated, but anti-inflammatories and analgesics are the most common culprits. It is not uncommon that FOP patients have skin rashes or hives due to the frequent use of drugs for pain relief or inflammation. Reactions to drugs can manifest as polymorphic erythema (with lesions on the skin and mucous membranes), fixed pigmented erythema, urticaria (hives), monomorphic acne or dyshidrosis, among others (Kang et al., 2019).
The use of prednisone, even for short periods of time, can also cause skin issues that include acne, skin and hair thinning, facial erythema, hirsutism, stria, or impaired wound healing. Some medications that are being tested in clinical trials also have influences on the skin. Palovarotene can cause dryness of the skin and mucous membranes, retinoid dermatitis, dry lips, and tendency to skin infections, among others.
Garetosmab can cause madarosis, acne, folliculitis, furunculus, carbuncles and abscesses.
Dermatologic conditions related to FOP itself – linked to the genetic mutation and signaling pathway
FOP is not just a bone disease. The way in which the genetic mutation affects the various body systems is still largely unknown. Dermatologic manifestations and their ties to FOP are still being investigated.
However, some common features have been anecdotally observed:
Flare-ups. During FOP flare-ups, the skin over the affected area may be tender, erythematous or warm (Pignolo et al., 2016). When heterotopic bone has formed, the skin can look glossy and feel hard upon touch, with loss of hair. It is important to avoid confusion with dermatologic diseases such as scleroderma, among others. Here again, we stress the diagnostic importance of the malformation of the great toes and the development of the progressive heterotopic ossification – signature clinical findings of classic FOP (Blaszczyk et al., 2003).
Scalp nodules. Scalp nodules may appear during childhood. They usually disappear spontaneously (McFarland et al., 1984).
Hair loss. Hair loss has occasionally been reported as a symptom of FOP, especially with severe FOP variants. Patients also report other hair-related issues (sparse eyebrows, less and lighter body hair. etc.).These additional features may also be under-reported symptoms of FOP (Pignolo et al., 2020).
Hypersensitivity to insect bites. This may be related to increased systemic mast cell reactivity in FOP (Pignolo et al., 2020).
Rare skin tumors. Merkel cell carcinoma (rare, aggressive, malignant primary cutaneous neuroendocrine tumor) has been noted rarely in some FOP patients – possibly related to lifelong corticosteroid use or sun exposure (Kang et al., 2019).
Other skin abnormalities. Other skin abnormalities ranging from skin discoloration to immune-related conditions such as seborrheic dermatitis have been noted in some FOP patients.
Clearly, there is a tremendous need to evaluate these dermatologic issues in the wider FOP community in order to determine the prevalence and risk of skin-related conditions in FOP.
Dermatologic conditions unique to each individual with FOP – genetic, environmental, and/or Occupational
Dermatological evaluation of a patient with FOP needs to be careful and comprehensive. Although the items above may be noted in FOP, their relative prevalence is unknown.
References
Blaszczyk M, Majewski S, Brzezinska-Wcislo L, Jablonska S. Fibrodysplasia ossificans progressiva. Eur J Dermatol 13: 234-237, 2003
Kang S, Masayuki A, Bruckner AL, Enk AH, Margolis DJ, McMichael AJ. Fitzpatrick's Dermatology, 9th edition, 2019
McFarland GS, Robinowitz B, Say B. Fibrodysplasia ossificans progressive presenting as fibrous scalp nodules. Cleve Clin Q 51: 549-552, 1984
Moriatis JM, Gannon FH, Shore EM, Bilker W, Zasloff MA, Kaplan FS. Limb swelling in patients who have fibrodysplasia ossificans progressiva. Clin Orthop Rel Res 336: 247-253, 1997
Pignolo RJ, Bedford-Gay C, Liljesthröm M, Durbin-Johnson BP, Shore EM, Rocke DM, Kaplan FS. The natural history of flare-ups in fibrodysplasia ossificans progressiva: a comprehensive global assessment. J Bone Miner Res 31: 650-656, 2016
Pignolo RJ, Wang H, Kaplan FS. Fibrodysplasia ossificans progressiva: a segmental progeroid syndrome.
Frontiers in Endocrinology 10 (Article 908): 1-8, 2020
5-17. Ingrown Toenails in FOP
Ingrown toenails, especially of the big toe, are common with people living with FOP. Patients with FOP are predisposed to ingrown toenail due to congenital foot and nail malformations, pressure from toe walking, improper nail trimming, poorly fitting shoes and retinoid medications. Ingrown toenails can lead to pain, discomfort and inability to bear weight on the affected foot as well as to an increased risk of infection.
Prevention of ingrown toenails includes cutting the nail at a 90 degree angle to the toe (and never too short; the nail must be above the nail bed) and removing pressure on the toe (using appropriate shoes).
Onychomycosis is a common fungal infection of the nail that can cause ingrown nails. Weakened immune system (use of steroids), trauma, and circulatory issues can facilitate the entrance of fungus into the nail. It is possible to observe changes in color and thickening of the nail that can be deformed and ingrown. The treatment for ingrown nails is antifungal drugs under the direction of a dermatologist.
Depending on the severity, various interventions might be necessary for ingrown toenail. For symptoms of mild pain and minimal inflammation, noninvasive procedures like changes in shoes, warm water soaks, antiseptics and over-the-counter topical antibiotics (like Bacitracin and Polymyxin B creams) might suffice. Oral antibiotics are used to treat superimposed bacterial infections. In more severe cases, referral to a dermatologist/wound specialist is warranted. In certain instances, removal of granulomatous tissue around the toenail by cauterization has been performed safely in FOP patients.
5-18. Fractures in FOP
Individuals with FOP are at increased risk of fractures of both the normotopic and heterotopic skeleton due to the increased risk of falls, immobility and prednisone use. Palovarotene (SOHONOS), approved in USA, Canada, and Australia has also been shown to decrease bone mineral density and could be associated with increased risk of fractures (SOHONOS prescribing information).
In FOP, fractures can occur in both normotopic and heterotopic bone (Pignolo et al., 2016). Fractures of heterotopic bone occur commonly and heal rapidly (Einhorn & Kaplan, 1994). Elevation, rest, splinting, and local application of ice are often helpful in controlling pain and swelling and may be supplemented by acute use of narcotic analgesia, if needed. Fractures of normotopic bone need to be carefully evaluated, as in any patient. Closed reduction and splinting is sufficient for most fractures. Open reduction or internal fixation is rarely warranted and can lead to rapid onset of HO. Healing may be delayed in osteoporotic bone.
In a retrospective study, Kaplan et al. (2023) examined the extent to which non-operative treatment of a fracture leads to an FOP flare-up, HO formation and loss of mobility. The authors asked: (1) What proportion of the fractures had radiographic evidence of union or nonunion (2) What proportion of patients had clinical symptoms of an FOP flare-up because of the fracture? (3) What proportion of fractures developed radiographic evidence of HO? (4) What proportion of patients lost movement after a fracture?
The authors retrospectively analyzed 31 patients (13 males, 18 females; ages 5 to 57 years, median age 22 years) who sustained 41 fractures of the normotopic skeleton that were treated nonoperatively from five continents over a 20-year period and followed for a minimum of 18 months after the fracture and for as long as 20 years. Clinical records for each patient were reviewed by the referring physician-author and the following data for each fracture were recorded: biological sex, ACVR1 gene pathogenic variant, age at time of fracture, fracture mechanism, fracture location, initial treatment modality, prednisone use at the time of the fracture as indicated in the FOP Treatment Guidelines for flare prevention, patient-reported flare-ups after the fracture, follow-up radiographs of the fracture if available, HO formation as a result of the fracture determined at a minimum of 6 weeks after the fracture, and patient-reported loss of motion. Postfracture radiographs were available in 76% (31 of 41) of fractures in 25 patients.
Radiographic healing was noted in 97% (30 of 31) of fractures at 6 weeks after the incident fracture. A painless nonunion was noted in one patient who sustained a displaced patellar fracture and HO. Three patients [7%; (3 of 41) of fractures] reported increased pain and/or swelling at or near the fracture site within several days after fracture immobilization that likely indicated a site-specific FOP flare-up. The same three patients reported a residual loss of motion one year after the fracture compared with their prefracture status. Ten percent (Three of 31) of the fractures for which follow-up radiographs were available developed HO. Patient-reported loss of motion occurred in 10% (4 of 41) of fractures. Two of the four patients reported noticeable loss of motion and the other two patients reported that the joint was completely immobile (ankylosed).
Most fractures treated nonoperatively in individuals with FOP healed with a paucity of flare-ups and HO and preservation of mobility, suggesting an uncoupling of fracture repair and HO, which are two inflammation-induced processes of endochondral ossification. These findings underscore the importance of considering non-operative treatment for fractures in individuals with FOP. Bone mineral density and bone quality evaluation in FOP is challenging due to bone deformity and HO. Consider bisphosphonate treatment in patients who have sustained a low trauma/fragility fracture after the fracture has healed. Notably, on patient with hip fracture treated with palovarotene still developed HO, although it is unclear if the amount of HO was lowered due to palovarotene treatment (Singh et al., 2020).
References
Einhorn TA, Kaplan FS. Traumatic fractures of heterotopic bone in patients who have fibrodysplasia ossificans progressiva. Clin Orthop Rel Res 308: 173-177, 1994
Lindborg CM, Al Mukaddam M, Baujat G, Cho TJ, De Cunto CL, Delai PLR, Eekhoff EMW, Haga N, Hsiao EC, Morhart R, de Ruiter R, Scott C, Seemann P, Szczepanek M, Tabarkiewicz J, Pignolo RJ, Kaplan FS. Most Fractures Treated Nonoperatively in Individuals With Fibrodysplasia Ossificans Progressiva Heal With a Paucity of Flareups, Heterotopic Ossification, and Loss of Mobility. Clin Orthop Relat Res 481: 2447-2458, 2023
Pignolo RJ, Bedford-Gay C, Liljesthröm M, Durbin-Johnson BP, Shore EM, Rocke DM, Kaplan FS. The natural history of flare-ups in fibrodysplasia ossificans progressiva: a comprehensive global assessment. J Bone Miner Res 31: 650-656, 2016
Singh S, Kidane J, Wentworth KL, Motamedi D, Morshed S, Schober AE, Hsiao EC. Surgical management of bilateral hip fractures in a patient with fibrodysplasia ossificans progressiva treated with the RAR-γ agonist palovarotene: a case report. BMC Musculoskelet Disord 2020 Apr 3;21(1):204
5-19. Temporomandibular Subluxations in FOP
Subluxations may be common in FOP due to joint malformations. Most are chronic and present from birth such as the common subluxation of the metatarsophalageal joint of the great toe. However, some may be more acute.
The absence of temporomandibular joint (TMJ) flare-ups and the suddenness of closure suggests a mechanical problem such as acute subluxation or dislocation of the jaw – rather than a flare-up. This is relatively easy to rule out with a CT scan of the TMJ. The key historical clue to subluxation of the TMJ in FOP is the absence of flare-up and the suddenness of closure. It is often painful, but may be less so in FOP. We now know that many FOP joints are malformed to various degrees - not just the great toe (Towler et al., 2020). The developmental anomalies of the jaw have received very little attention.
The TMJ in FOP patients is prone to develop problems. This may be due to developmental abnormalities as mentioned above. The TMJ in FOP does not form normally. The mandibular condyles are often flat and the menisci (similar to the knee) are often malformed. We rarely ask FOP patients to open their mouth maximally, but if we did, we would see that this does not occur normally. We assign a CAJIS of zero if there is good functional opening (but not maximal because we don’t test it).
If the TMJ is not subluxed or dislocated and if it is fused, do not try to force it open. Most patients do well with multiple small meals and puréed foods - not ideal - but bearable. Very few need a naso-gastric tube, but those who do tend to tolerate it well. The overbite of FOP can often help with access to eating. If necessary, back teeth can be removed to help with dental access, eating, and fear of vomiting.
References
Towler OW, Shore EM, Kaplan FS. Skeletal malformations and developmental arthropathy in individuals who have fibrodysplasia ossificans progressiva. Bone 2020 Jan; 130:115116
5-20. Nutrition, Calcium & Vitamin D Guidelines in FOP
Patients with FOP are at high risk of nutritional and vitamin deficiencies due to multiple factors, including but not limited to gastrointestinal issues, ankylosed jaw, nausea and poor appetite (Mantick et al., 2018).
Adequate nutrition is especially important in FOP patients to prevent severe weight loss, Superior Mesenteric Artery (SMA) syndrome and pressure ulcers. For patients with an ankylosed jaw, we recommend referral to a nutritionist; in extenuating situations, patients might need a feeding tube. Ideal weight will be difficult to determine due to inaccurate height measurements and BMI calculation, but we recommend monitoring weight and addressing unintentional weight loss (>5% of weight over 6-12 months) as soon as it is noted (Wong et al., 2014).
FOP patients can also have vitamin deficiencies, including vitamin D deficiency, especially if they spend most of their time indoors. Calcium intake could also be suboptimal due to the misconception that it could further worsen heterotopic ossification.
We recommend that patients obtain the recommended dietary allowance (RDA) of calcium as per Institute of Medicine 2011 guidelines, which would be 1,000 mg of elemental calcium daily for adults, age 18-50 years old. We recommend that calcium intake be obtained using diet (example milk, cheese, yoghurt or food items fortified with calcium like orange juice and almond milk) and limit calcium supplements to no more than 600 mg daily. Excessive calcium intake, especially in the form of supplements, can increase the risk of kidney stones which patients with FOP are prone to develop. It is also important for patients with FOP to maintain adequate hydration to avoid kidney stones.
We recommend that patients also obtain the RDA for vitamin D as per the Institute of Medicine 2011 guidelines, which would be 600 units of Vitamin D3 daily for adults, age 18-50 years old. We recommend measuring serum 25-OH-vitamin D and repleting as needed to maintain a level >20-30 ng/dl. Low 25-OH- vitamin D levels in the serum are associated with osteomalacia/rickets that could lead to increased risk of normotopic bone fracture. In addition, low vitamin D levels increase the risk of hypocalcemia after administration of intravenous bisphosphonates.
References
Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC. The National Academies Press, 2011
Mantick N, Bachman E, Baujat G, Brown M, Collins O, De Cunto C, Delai P, Eekhoff M, Zum Felde R, Grogan DR, Haga N, Hsiao EC, Kantanie S, Kaplan FS, Keen R, Milosevic J, Morhart R, Pignolo RJ, Qian X, Di Rocco M, Scott C, Sherman A, Wallace M, Williams N, Zhang K, Bogard B. The FOP connection registry: Design of an international patient-sponsored registry for Fibrodysplasia Ossificans Progressiva. Bone 109: 285-290, 2018
Smith B, Hakim-Zargar M, Thomson J. Low body mass index: a risk factor for superior mesenteric artery syndrome in adolescents undergoing spinal fusion for scoliosis. J Spinal Disord Tech 22: 144-148, 2009
Wong CJ. Involuntary weight loss. Med Clin North Am 98: 625-43, 2014
5-21. Preventive Oral Healthcare in FOP
Individuals with FOP have developmental anomalies of the temporomandibular joints (TMJs) (Connor & Evans, 1982; Renton et al., 1982; Carvalho et al., 2011). Spontaneous or post-traumatic ankylosis of the TMJs is common in FOP and leads to severe disability with resultant difficulties in eating and poor oral hygiene. Great care must be taken not to provoke flare-ups of the TMJ (Luchetti et al., 1996). Ossification of the masseter muscle results in the smallest mouth opening and ossification of the zygomatic arch results in the largest mouth opening. Thus, the location of the HO impacts the extent of the mouth opening (Schoenmaker et al., 2022).
Managing the risk of any form of oral disease is essential in patients with FOP, especially during childhood years (Young et al., 2007). Preventing development of caries or periodontal disease is crucial to prevent long-term dental and oral complications in patients with FOP.
Fluoridation of water is suggested for all patients who have FOP. The use of high dose fluoride toothpaste (once an individual can spit) is recommended along with use of fluoride gels and rinses to help prevent the need for restorative dental care (Hujoel et al., 2018). Children who are unable to spit can use a rice size grain of normal fluoridated toothpaste.
Frequent flossing and brushing are necessary in patients with FOP, as in anyone, but may be difficult due to limited jaw opening as FOP progresses. Ultrasonic, electric, or battery-powered toothbrushes with small heads, water picks and floss wands may also help with dental hygiene. If these are suggested, please check with your oral health professional on appropriate and best technique to use these devices.
Patients with FOP who have sufficient mouth opening can be treated with normal dental instruments as in unaffected individuals, but great care must be exercised to prevent overstretching of the TMJs during dental procedures. In patients who have ankylosed TMJs, professional instrumentation and special toothbrushes may be helpful, but are often limited to use on the buccal surfaces. Antimicrobial and fluoride rinses may be the only method to reach the lingual and palatal surfaces (Nussbaum et al., 2005).
For patients who have frank cavitation, the use of 38% Silver Diamine Fluoride (SDF) is recommended as a non-surgical treatment approach. Use of glass ionomer sealants in both primary and permanent dentition is highly recommended (Slayton et al., 2018). SMART (Silver modified atraumatic technique) technique may also be used. Partial caries removal with no anesthesia and placement of a glass ionomer restoration can often suffice until a primary molar exfoliates (Innes et al., 2016; Wong et al., 2017).
For FOP patients with frank cavitation requiring minor restorative procedures that can be performed in a dental office using infiltration or inter-ligamentary anaesthesia or lasers (but never mandibular blocks), treatment can be accomplished without the use of pre-procedure prednisone. This approach requires pre- procedure assessment of maximum mouth opening and placement of a molt or McKesson mouth prop 3-4 mm less than the maximum opening. Frequent intermissions are beneficial to limit stress and injury to soft tissue. Multiple appointments are possible. However, two-week intervals between appointments are suggested.
In patients who have difficulty maintaining oral hygiene or who have gingivitis, chlorhexidine rinses are encouraged. Chlorhexidine is normally used once daily for 6 weeks and then once daily for one week a month. Long-time use of chlorhexidine can result in staining of teeth.
Patients with a high risk of dental caries or difficulty in maintaining good oral hygiene may request that their dentist professionally apply fluoride varnish or a bio-erodible and fluoride resin. Fluoride varnish should be applied 3-4 times a year – often in conjunction with silver diamine fluoride (SDF) (Gao et al., 2016). There is good evidence that these modalities are effective in inhibiting tooth demineralization (Donly, 2003; Lin et al., 2009; Pitts & Zero, 2016; Slayton et al., 2018). A minimum three months’ professional follow-up for these patients is suggested. For patients who have early stages of interproximal lesion development use of a resin infiltration technique plus the use of a fluoride varnish and/or SDF is suggested (Slayton et al., 2018).
Saliva testing is highly recommended for patients with FOP. Qualitative and quantitative evaluations should be done. Salivary flow both resting and stimulated, and buffering capacity should be performed as a baseline and if a patient is on any medication. Many of the FOP clinical trial drugs will cause dry mouth. If a patient has inadequate resting and stimulated saliva flow, habitual rinsing with water after any food intake is helpful. Saliva substitutes may also be helpful. For patients who may have a low pH or inability to buffer use of MI Paste, Tooth Mousse or Amorphous Casein Phosphate – ACP may be beneficial as will use of xylitol containing gum and or rinses (Pitts & Zero, 2016). Other products for MIX (medication induced xerostomia) may also be available dependent on country of origin.
Any activity that can minimize the risk of oral health degradation should be implemented. The focus on this section is the pharmacologic treatment of disease rather than surgical. This is especially important in someone with FOP where doing invasive dental treatment can trigger flare-ups.
The following is a list of such suggestions:
Pediatric patients under 3 years of age:
First visit to an oral health professional - by latest, one year of age.
Begin oral sanative cleaning after each breast or bottle feeding.
Use rice size grain of fluoridated toothpaste especially if living in a non-fluoridated area.
Minimize use of soft cariogenic foods. Sugar is ubiquitous and present in many foods commonly given to children. Yoghurt is a good example of a food that may contain an excess of sugar.
Fluoride varnish suggested twice annually for children at high risk.
Pediatric patients over 3 years of age:
Assess risk of development of oral disease, and if high risk 3 monthly professional appointments suggested. If this is not possible, begin use of high dose fluoride toothpaste (rice size grain) and frequent daily brushing and flossing.
Pediatric patients over 5 years of age (who can spit):
Begin use of fluoride toothpaste – if high risk, use high dose fluoride toothpaste and provide professionally applied fluoride varnish a minimum of 2-3 times a year.
Continue to monitor and direct diet that is low in sugar.
Most children do not have the ability to floss effectively until 10-11years of age and would benefit from assistance. Floss aids can be used. However, parent should be aware of cross contamination and be sure to rinse a floss wand after flossing each tooth area.
Monitor nasal or mouth breathing – snoring or grinding. Mouth breathing can cause altered oral facial development and increase risk for oral disease.
Patients with normal mouth opening can be treated with normal dental instruments however, care should be taken to prevent overstretching of the TMJ and musculature. Trauma and anoxia are known triggers for FOP episodes.
Orthodontics – (see section on orthodontics)
For patients who have carious lesions consider the use of SDF, partial caries removal concept (smart restorations) with the use of glass ionomers and Hall Crowns. None of these applications require the use of local anesthesia.
Adolescents and Adults:
In patients who have normal mouth opening and are at low risk – regular care, brushing, flossing, fluoridated toothpaste, and dietary monitoring are suggested. Bi-annual professional visits should be sufficient.
Orthodontics (see section on orthodontics)
Any patient who is at high risk for oral disease should be seen at least every three months, if possible.
For patients with any decrease in mouth opening, jaw dysfunction, or difficulty in maintaining good oral hygiene, the following are recommended:
High dose fluoride toothpaste
Saliva testing – (qualitative and quantitative) and appropriate intervention based on results
Silver diamine fluoride (SDF) 38% application on any beginning lesion (cavity) followed by placement of a high fluoride containing glass ionomer fluoride, xylitol rinses
Monitor and minimize sugar intake
Use of chlorhexidine rinses or varnish
Fluoride varnishes
For minimal interproximal lesions – SDF – and or resin infiltration techniques and fluoride varnish
Brushing and flossing aids. Electric toothbrushes with small heads, water pics and floss wands are examples.
The electric toothbrush should be used appropriately according to the specific recommendations of each brand. However, the brushing guidelines are generally similar across all brands: one should let the toothbrush perform the brushing motions and simply position the brush at the area to be cleaned without making additional circular or back-and-forth movements.
For patients with partial or total ankylosis of the temporomandibular joint, especially those who push food to one side of the mouth to eat, the recommendations are to rinse the mouth after each meal and to wait at least 30 minutes after rinsing before brushing the teeth. It is now understood that the quality of the microbiome and acidity of the oral cavity significantly impact the enamel and gums.
CMP (MI paste)
Xylitol containing gum.
References
Carvalho DR, Farage L, Martins BJ, Speck-Martins CE, Craniofacial findings in fibrodysplasia ossificans progressiva: computerized tomography evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 111: 499-502, 2011
Connor JM & Evans DA. Extra-articular ankylosis in fibrodysplasia ossificans progressiva. Br J Oral Surg 20: 117-121, 1982
Donly KJ. Fluoride Varnishes. J Calif Dental Assoc 31: 217-219, 2003
Gao SS, Zhao IS, Hiraishi N, Duangthip D, Mei ML, Lo ECM, Chu CH. Clinical trials of silver diamine fluoride in arresting caries among children: A Systematic Review. JDR Clin Trans Res 1: 201-210, 2016
Hujoel PP, Hujoel MLA, Kotsakis GA. Personal oral hygiene and dental caries: A systematic review of randomised controlled trials. Gerodontology May, 2018
Innes NPT et al: Managing carious Lesions: Consensus recommendations and terminology. Advances in Dental Research 28: 49-57, 2016
Lin R, Hildebrand T, Donly KJ. In vitro remineralization associated with a bio-erodible fluoridated resin and a fluoride varnish. Am J Dentist 22: 203-205, 2009
Luchetti W, Cohen RB, Hahn GV, Rocke DM, Helpin M, Zasloff M, Kaplan FS. Severe restriction in jaw movement after route injection of local anesthetic in patients who have fibrodysplasia ossificans progressiva. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 81: 21-25, 1996
Nussbaum BL. Dental care for patients who are unable to open their mouths. Dental Clin North Am 53: 323-328, 2009
Nussbaum BL, Grunwald Z, Kaplan FS. Oral and dental healthcare and anesthesia for persons with fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab 3: 239-242, 2005
Pitts N, Zero D. White paper on dental caries prevention and management: fdiworldental.org/media/93783/2016-fdi_cpp-white_paper.pdf, 2016
Renton P, Parkin SF, Stamp TC. Abnormal temporomandibular joints in fibrodysplasia ossificans progressiva. Br J Oral Surg 230: 31-38, 1982
Schoenmaker T, Dahou Bouchankouk A, Özkan S, Gilijamse M, Bouvy-Berends E, Netelenbos C, Lobbezoo F, Eekhoff EMW, de Vries TJ. Limitations of Jaw Movement in Fibrodysplasia Ossificans Progressiva: A Review. Front Med (Lausanne). 2022 Mar 22;9:852678
Wong A, Subar PE, Young DA. Dental Caries: An update on dental trends and therapy. Adv Pediatr 64: 307-330, 2017
Young JM, Diecidue RJ, Nussbaum BL. Oral management in a patient with fibrodysplasia ossificans progressiva. Spec Care Dentist 27: 101-104, 2007
5-22. Extraction of Wisdom Teeth
Wisdom teeth may be considered for extraction if:
The wisdom teeth have erupted and if they are in close proximity to the adjacent molar and certain nerves among other structures.
It is determined that the wisdom teeth may present future complications and or is partially erupted.
WARNING: Wisdom teeth extraction in FOP patients with normal jaw function always carries the risk of flare-ups and or ankylosis of the jaw, even with assiduous precautions. Thus, the relative risks and benefits of early removal of wisdom teeth must be considered on an individual basis.
Technique for removal:
If the patient has a fused jaw, a buccal approach may be used for the extraction. If the mouth opening is normal, removal of the wisdom teeth may be performed using a normal approach.
Removal of teeth for eating and emesis:
For a patient with a fused jaw, removing the premolars or molars unilaterally in both the upper and lower jaw might be considered and would allow both feeding and emesis to occur. It is also important to take into consideration the state of the posterior teeth, and ability to maintain cleaning procedures. Sometimes bilateral extractions might be indicated.
Antibiotics:
Prophylaxis with antibiotics is always suggested but must be carefully reviewed as certain antibiotics are contraindicated in patients who are enrolled in clinical trials. Please check with the primary physician to ascertain if the patient is enrolled in a clinical trial and what would be the appropriate antibiotic to use.
Corticosteroids:
Corticosteroid prophylaxis is imperative with wisdom teeth extraction in individuals with FOP as outlined in Section-2
5-23. Orthodontics & FOP
Most people seek orthodontic care for aesthetic and functional reasons. For the FOP population, self-image is as important as in the general population. Orthodontic therapy can be safely performed on patients with FOP who have normal or nearly normal oral opening (Luchetti et al., 1996).
Patients who have FOP often develop mandibular hypoplasia with a maxillary overbite and, therefore, orthodontic therapy may be considered (Hammond et al., 2012). However, many patients find that the overbite provides a means of access for eating as well as for oral and dental hygiene. Posterior and anterior dental cross-bites can influence the TMJs and should be corrected. For children with functional TMJs and with anterior open bites that are less than 15 mms, orthodontics is not recommended as the overbite will facilitate nutrition and subsequent dental care if the TMJ does eventually ankylose.
When orthodontic care is considered, brief appointment times should be used to lessen stress on the TMJs.
The use of non-extraction therapy is also recommended. To prevent the need for extractions in FOP patients, it may be advisable to align the anterior segments for aesthetics, leaving posterior dental crowding untreated. Crowded posterior teeth may be a better alternative than the risks of flare-up and TMJ ankylosis that can accompany an extraction (Levy et al., 1999). Use of Invisalign orthodontics may be advantageous as forces generated are far less than with traditional orthodontics and daily maintenance of oral health is considerably easier. Intra-oral scanning techniques are now available that can be used especially useful for patients who have difficulty with impressions. Data on the relation between orthodontic forces and tooth movement and HO is lacking. Most case reports identify that orthodontics can be performed in patients with FOP.
References
Hammond P, Suttie M, Hennekam RC, Allanson J, Shore EM, Kaplan FS. The face signature of fibrodysplasia ossificans progressiva. Am J Med Genet 158A: 1368-1380, 2012
Levy CE, Berner TF, Bendixen R. Rehabilitation for individuals with fibrodysplasia ossificans progressiva.
Clin Rev Bone Miner Metab 3: 251-256, 2005
Luchetti W, Cohen RB, Hahn GV, Rocke DM, Helpin M, Zasloff M, Kaplan FS. Severe restriction in jaw movement after route injection of local anesthetic in patients who have fibrodysplasia ossificans progressiva. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 81: 21-25, 1996
Slayton RL, Urquhart O, Araujo MWB, Fontana M, Guzmán-Armstrong S, Nascimento MM, Nový BB, Tinanoff N, Weyant RJ, Wolff MS, Young DA, Zero DT, Tampi MP, Pilcher L, Banfield L, Carrasco- Labra A. Evidence -based clinical practice guideline on non-restorative treatments for carious lesions. A report from the American Dental Association. JADA 149: 837-849, 2018
5-24. Submandibular Flare-ups in FOP
Submandibular flare-ups are among the most concerning of all flare-ups due to their potential for causing difficulties in breathing and swallowing. Rarely, however, does jaw movement become affected by flare- ups isolated to this region. Due to their significance for vital functions, submandibular flare-ups warrant special attention (Janoff et al., 1996; Leavitt et al., 2009).
A comprehensive study of submandibular swelling in patients with FOP was undertaken by Janoff et al. and published in 1996. Twelve (11%) of 107 patients who were studied had submandibular HO that was mistaken initially in seven of the patients for mumps, angioneurotic edema, abscess, mononucleosis, or neoplasm. Two male patients and ten female patients ranging in age from 6-47 years (mean: 21 years) were studied. Ten patients survived following assiduous precautionary measures. One patient who required emergency tracheostomy and ventilatory support also survived. Another patient died of inanition from chronic swallowing difficulty.
An effective treatment program includes early identification of the submandibular flare-up, nutritional support, and glucocorticoid therapy. Submandibular swelling in patients who have FOP can be a medical emergency and requires intensive precautionary measures to avoid catastrophic clinical deterioration. These measures include avoidance of lesional manipulation, airway monitoring, and aspiration precautions. Submandibular swelling should be recognized as a variable feature of FOP with important potential additional clinical consequences (Janoff et al., 1996).
An isolated case report of submandibular swelling in a patient with FOP (Leavitt et al., 2009) notes that submandibular flare-ups can be troublesome and endanger the airway or swallowing. The authors note that patients with FOP often have limited jaw movement and associated dental problems and that dental professionals often misdiagnose FOP patients with submandibular flare-ups as having dental abscesses or odontogenic infection. Making matters worse, any manipulation of the soft tissues of the mouth, or the head, or neck will hasten inflammation and worsen the clinical problem of flare-ups in the submandibular region.
Patients with submandibular flare-ups of FOP must be properly diagnosed so that intensive precautionary measures to avoid catastrophic clinical deterioration can be instituted. An important challenge in the care of patients with a submandibular flare-up of FOP is to effectively manage the acute symptoms and associated discomfort while avoiding meddlesome intervention that will likely exact the lesion.
Based on our extensive clinical experience, we recommend the following:
All physicians who treat patients with FOP should be aware that acute submandibular swelling may be a manifestation of the disease at any age and can occur during childhood in patients with rapid disease progression.
Diagnosis of FOP should be communicated to the attending physician so that the acute submandibular lesion can be managed in the context of the patient’s underlying disease.
Manipulation of acute lesions must be avoided because even minor trauma can lead to catastrophic exacerbation with airway compromise.
Patients having an acute submandibular flare-up should sleep with their head elevated, if possible, to decrease the risk of airway obstruction.
Patients with an acute submandibular flare-up should be monitored closely during the acute swelling phase and should be hospitalized immediately in the event of pending obstruction of the airway.
Food should be pureed or semi-solid. Clear liquids often provoke choking episodes during submandibular flare-ups as the involved muscles are those that move the tongue at the floor of the mouth.
Patients should be encouraged to eat frequently to minimize weight loss.
Additional high calorie food supplements should also be considered.
Assiduous precautions should be undertaken to prevent food aspiration.
High dose oral glucocorticoids should be considered in the very early treatment of acute submandibular flare-ups. The glucocorticoid of choice is prednisone, and the dose is 2 mg/kg of bodyweight (up to 100 mg) taken per oral once daily for four days at the earliest sign of an acute flare-up. While these flare-ups do not last longer than flare-ups at other locations, the functional consequences of flare-ups in the submandibular region may warrant a longer glucocorticoid treatment course or a second course of steroids if severe soft tissue swelling recurs after cessation of steroid use. If a second four-day course of high dose glucocorticoids is used, the steroids should be tapered slowly over the next two to three weeks. Following discontinuation of the glucocorticoid therapy, non-steroidal anti-inflammatory medications or a COX-2 inhibitor can be considered for the next 6-8 weeks.
References
Janoff, HB, Zasloff MA, Kaplan FS. Submandibular swelling in patients with fibrodysplasia ossificans progressiva. Otolaryngol Head Neck Surg 114: 599-604, 1996
Leavitt BD, Teeples TJ, Viozzi CF. Submandibular space swelling in a patient with fibrodysplasia ossificans progressiva: a diagnostic dilemma. J Oral Maxillofac Surg 67: 668-673, 2009
5-25. Swallowing and FOP
Swallowing may be among the most difficult of problems for some individuals with FOP. The swallowing mechanism is complex and involves cranial and peripheral nerves as well as the extrinsic muscles of the tongue (which are often involved in flare-ups of the submandibular space) as well as rare FOP involvement of the skeletal muscles of the upper third of the esophagus. As a result, chronic dysphagia (difficulty with swallowing) may manifest with mechanical difficulty in swallowing, aspiration of food, coughing, recurrent pneumonia (due to aspiration), difficulty in managing secretions, extreme discomfort, anxiety, and fear of eating as well as progressive weight loss and resultant malnutrition.
If chronic swallowing problems exist, swallowing tests as well as consultation with ENT, and FOP specialists as well as FOP-Anesthesia specialists should be obtained. Gastrointestinal feeding tubes (G- Tubes) may be necessary to bypass the severely dysregulated swallowing mechanism and are generally well-tolerated.
References
Janoff HB, Zasloff MA, Kaplan FS. Submandibular swelling in patients with fibrodysplasia ossificans progressiva. Otolaryngol Head Neck Surg 114: 599-604, 1996
5-26. Dental Anesthesia in FOP
Patients with Fibrodysplasia Ossificans Progressiva (FOP), like all other patients, should be able to receive dental care under the best possible anesthetic conditions, ensuring pain-free treatment.
Therefore, local dental anesthesia is highly recommended making it clear that Mandibular blocks are forbidden as they will lead to ossification of the pterygoid muscles and rapid ankylosis of the TMJ (Luchetti et al., 1996).
Infiltration anesthesia is difficult in the mandibular posterior molar areas of permanent teeth. Successful anesthesia in mandibular primary teeth can be achieved by infiltration through the alveolar bone. Use of a hard tissue laser can preclude the use of anesthesia especially in small lesions.
Interligamentary infiltration may be helpful, if performed carefully. However, in some patients, this type of local anesthesia may not be possible. General anesthesia (GA) may be needed for dental care in patients with FOP (Nussbaum et al., 1996; Nussbaum et al., 2005).
Cervical spine fusion, ankylosis of the TMJ, thoracic insufficiency syndrome, restrictive chest wall disease, and sensitivity to oral trauma complicate airway management and anesthesia and pose life-threatening risks in individuals with FOP.
A retrospective chart review was conducted at one institution of patients with FOP who underwent GA for treatment of complex dental procedures (Kilmartin et al., 2014).
Even for simple procedures, if the patient does not open his or her mouth sufficiently, even vigilant care can be a source of operative insecurity (ingestion/inhalation), forcing the team to perform care under general anesthesia.
Thirty patients underwent 42 general anesthetics. GA was induced most commonly after the airway was secured by an awake fiberoptic intubation. GA can be administered safely to patients with FOP for dental procedures with attention to airway management and perioperative care using a multidisciplinary approach. An awake nasal fiberoptic intubation should be considered the first choice for airway management. Most patients can be discharged home on the same day as their dental procedure.
This large case series demonstrates that GA for dental procedures can be safely accomplished in patients with FOP using a multidisciplinary approach. Because of the difficult airway management reports in the literature and the routine use of numerous medical specialties, we recommend that patients with FOP are cared for at an institution where a multidisciplinary approach is possible.
An anesthesiologist should evaluate the patient preoperatively, preferably before the day of the procedure. An otolaryngologist should be immediately available during the procedure to assist with airway management and perform an emergency tracheostomy if needed. A dentist and an oral maxillofacial surgeon should be involved in each case so that a comprehensive oral rehabilitation with tooth extractions can occur under one GA setting, which is safer and more convenient for the patient.
Other medical practitioners, such as pediatricians, family medicine physicians, cardiologists, pulmonologists, and intensivists, may be required.
An awake nasal fiberoptic intubation should be considered the first choice for airway management. There are several reasons for choosing GA with an endotracheal tube in this case series.
First, routine injections of local anesthetic for dental procedures, especially mandibular blocks, should be avoided because they can precipitate flare-ups and cause fusion of the TMJ.
Second, oral access can be difficult if a patient has decreased mouth opening. If a tooth fragment were dropped in the mouth of an unintubated patient with a fused jaw, it could be impossible to retrieve. An endotracheal tube provides needed airway protection.
Third, the dentist and the oral and maxillofacial surgeon may need Trendelenburg positioning for long periods of time. This positioning may cause patient discomfort and result in respiratory compromise, especially in patients with pre-existing pulmonary disease.
Positioning consideration is essential; patients’ bodies are often fused in a rigid position. All pressure points must be padded, and the neck supported. If a patient’s cervical spine is fused in flexion, a steep Trendelenburg positioning is often needed for adequate dental exposure. Positioning considerations for Trendelenburg include padding the patients’ shoulders and securing the patients to the bed to ensure that their bodies do not shift on the operating room table.
Fourth, we recommend the administration of perioperative corticosteroids to prevent and mitigate flare- ups. A 4-day perioperative corticosteroid course should be administered according to the current guidelines and begin before the start of the procedure.
In summary, a multidisciplinary approach to the perioperative management of patients with FOP should be the standard of care. Patients should be pretreated with corticosteroids and carefully positioned for surgery. GA can be safely administered to FOP patients for dental procedures; an awake nasal fiberoptic intubation is suggested as the first choice for airway management. Most patients can be discharged home on the same day as their dental procedure.
For pre-op consultation on GA for FOP patients, please contact:
Zvi Grunwald, M.D.
The James D. Wentzler Professor and Chairman Emeritus Department of Anesthesiology
Thomas Jefferson University and Hospitals 111 South 11th Street, Suite G-8490 Philadelphia, PA 19107, USA
Tel: 215-955-6161
Cell: 215-206-7362
Fax: 215-923-5507
Email: zvi.grunwald@jefferson.edu
References
Kilmartin E, Grunwald Z, Kaplan FS, Nussbaum BL. General anesthesia for dental procedures in patients with fibrodysplasia ossificans progressiva: a review of 42 cases in 30 patients. Anesth Analg 118: 298-301, 2014
Luchetti W, Cohen RB, Hahn GV, Rocke DM, Helpin M, Zasloff M, Kaplan FS. Severe restriction in jaw movement after route injection of local anesthetic in patients who have fibrodysplasia ossificans progressiva. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 81: 21-25, 1996
Nussbaum BL, Grunwald Z, Kaplan FS. Oral and dental healthcare and anesthesia for persons with fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab 3: 239-242, 2005
Nussbaum BL, O’Hara, I, Kaplan FS. Fibrodysplasia ossificans progressiva: report of a case with guidelines for pediatric dental and anesthetic management. ASDC J Dent Child 63: 448-450, 1996
5-27. General Anesthesia in FOP
General Considerations
Cervical spine fusion, ankylosis of the TMJ, thoracic insufficiency syndrome, restrictive chest wall disease, and sensitivity to oral trauma complicate airway management and anesthesia and pose life- threatening risks in individuals with FOP. Due to the challenges of airway management, general anesthesia (GA) is needed for most procedures in FOP patients and is an exceedingly complex issue (Kilmartin et al., 2014)
GA can be administered safely to patients with FOP with attention to airway management and perioperative care using a multidisciplinary approach. An awake nasal fiberoptic intubation should be considered the first choice for airway management.
Because of the difficult airway management reports in the literature and the routine use of numerous medical specialties, we recommend that patients with FOP are cared for at an institution where a multidisciplinary approach is possible.
An anesthesiologist should evaluate the patient preoperatively, preferably before the day of the procedure. An otolaryngologist should be immediately available during the procedure to assist with airway management and perform an emergency tracheostomy if needed. Other medical practitioners, such as pediatricians, family medicine physicians, cardiologists, pulmonologists, and intensivists, may be required.
In summary, a multidisciplinary approach to the perioperative management of patients with FOP must be the standard of care. Patients should be pretreated with corticosteroids and carefully positioned for surgery. An awake nasal fiberoptic intubation is suggested as the first choice for airway management. Even minor procedures should be scheduled to a major hospital setting, however same day discharge may be considered for individual patients. If postoperative admission is considered follow up should be assigned to an intensive care setting.
For pre-op consultation on GA for FOP patients, please contact:
Zvi Grunwald, M.D.
The James D. Wentzler Professor and Chairman Emeritus Department of Anesthesiology
Thomas Jefferson University and Hospitals 111 South 11th Street, Suite G-8490 Philadelphia, PA 19107, USA
Tel: 215-955-6161
Cell: 215-206-7362
Fax: 215-923-5507
Email: zvi.grunwald@jefferson.edu
Pre-operative preparation
A preoperative visit and meeting with the anesthesiologist prior to the date of surgery is crucial for conducting a safe and smooth general anesthesia course. The anesthesiologist should become familiar with FOP, learn about the extent of the disease affecting the individual patient, and carefully plan the perioperative anesthesia care.
In the case of a patient presenting with advanced disease, thoracic insufficiency syndrome, significant ankylosis of multiple joints, and/or limited mobility and co-morbidities, the anesthesiologist should plan to assemble a multidisciplinary team consisting at least of an anesthesiologist and a surgeon experienced in emergency airway management. Sharing the anesthesia plan with the patient and the family is useful to diffuse apprehension and foster cooperation on the day of surgery.
The special care and the skills required by the anesthesiologist to treat an FOP patient may not be available at peripheral locations or community hospitals. In these situations, the referring physician, the patient, and the family should seek referral to a major medical center with practitioners who are skilled in the care of FOP patients and the anesthesiologist can perform nasal fiberoptic intubation of the trachea.
We recommend the administration of perioperative corticosteroids to prevent and mitigate flare-ups associated with most surgical procedures. A four-day postoperative corticosteroid course should be administered according to the current guidelines and begin on the day of surgery - before the start of the procedure.
Intra-operative management
Positioning: Positioning consideration is essential as patients’ bodies are often fused in a rigid position. All pressure points must be padded, and the neck supported. Positioning considerations for Trendelenburg include padding the patients’ shoulders and securing the patients to the operating room table to ensure that their bodies do not shift during the surgical procedure. Extra padding will help minimize soft tissue trauma during the surgical procedure. The operating room table should be adjusted according to patient’s needs.
Monitoring: Routine monitoring is required for most surgical procedures (ECG, non-invasive blood pressure, pulse oximetry, end-tidal CO2, and temperature). Significant co-morbidities, lengthy surgical procedures, or a compromised cardio-respiratory system may require additional monitors. In patients whose upper limbs are ankylosed in adduction and flexion, the application of a blood pressure cuff may be difficult or impossible. The cuff may be applied on the lower extremity. A thin layer of padding under the cuff may reduce the impact of the frequent inflations of the cuff on the extremity.
Intravenous access: Careful venipuncture and short-term application of a tourniquet are usually benign. Indwelling intravenous or arterial catheters may rarely lead to the formation of an ossified tract at the site of insertion. Therefore, the smallest intravenous catheter appropriate for the procedure should be selected for insertion.
General anesthesia and sedation: The administration of general anesthesia and the maintenance of a patent airway are particularly challenging matters in patients who have FOP, and should be planned with exacting care. Guidelines for general anesthesia have been reported (Kilmartin et al., 2014).
Physicians and patients may be tempted to use sedation techniques and perform minor surgical procedures at an office-based or out-patient facility. The risks of catastrophic airway emergencies far outweigh the potential benefits of this option. Procedures should be performed only at facilities equipped with the skills and support systems necessary for a safe outcome. For patients with advanced disease, it is recommended that even minor procedures (colonoscopies, dental procedures) be performed at a major medical center under general anesthesia with a secured airway by nasal fiberoptic endotracheal intubation.
Patients who can open the mouth: In patients who are able to open the mouth, it is imperative to avoid over-stretching the TMJ during direct laryngoscopy. Careful positioning of the patient and the head, maintenance of a sniffing position and the use of a Glidescope (GlideScope® Video laryngoscope; GVL®) with minimal mouth opening is one approach of securing the airway. The use of a laryngeal mask airway (LMA) should be seriously questioned. Establishing endotracheal intubation under emergency conditions is extremely challenging resulting in significant morbidity and mortality (Reviewed in Kilmartin et al., 2014).
In cases where adequate mouth opening is questionable, an awake fiberoptic nasotracheal intubation is recommended.
Patients who cannot open the mouth: In patients who present with fusion of the cervical vertebrae, limited mouth opening, or ankylosis of the TMJ, oral access for endotracheal intubation is not possible. For these patients, an awake fiberoptic nasotracheal intubation under light sedation is recommended. Dexmedetomidine may be a reasonable choice. This should be performed by well- trained anesthesia teams who are experienced with this type of procedure (Tumolo et al., 2006; Kilmartin et al., 2014). The team should consist of two experienced anesthesiologists. A back-up surgeon (usually an otorhinolaryngologist) experienced in performing tracheostomies should be present with an immediately available tracheotomy tray. Nasal fiberoptic endotracheal intubation is performed with attention to administration of vasoconstrictors to the nose and the use of lubricated nasopharyngeal tubes starting with a small one and increasing the diameter up to 32-34 Fr.
All patients who undergo intubation should receive prophylactic steroids (methylprednisolone 50 mg iv q6 hrs, followed by oral prednisone taper once tolerating oral intake) to decrease the risk of fatal airway swelling and edema.
Perioperative pain management and regional anesthesia
Most patients with FOP present with advanced ossifications at the thoraco-lumbar area precluding access to spinal or epidural analgesia. Furthermore, such an approach can lead to catastrophic flare- ups.
Patients who are receiving preoperative pain medications including opioids may present significant intraoperative management challenge to the anesthesiologist. The use of intravenous ketamine should be considered. The use of opioids should be minimized to avoid postoperative respiratory depression. The use of acetaminophen and nonsteroidal anti-inflammatory medication is advised after discussion with the surgeon. Postoperative pain management should be accomplished with intravenous medications. Patients using patient controlled analgesia (PCA) devices should receive supplemental oxygen with careful monitoring of oxygenation at all times. Oral medications should be prescribed to patients who can open the mouth.
All questions regarding general anesthesia should be directed to Dr. Zvi Grunwald (please see above).
References
Kilmartin E, Grunwald Z, Kaplan FS, Nussbaum BL. General anesthesia for dental procedures in patients with fibrodysplasia ossificans progressiva: a review of 42 cases in 30 patients. Anesth Analg 118: 298-301, 2014
Tumolo M, Moscatelli A, Silvestri G. Anaesthesia management of child with fibrodysplasia ossificans progressiva. Br J Anaesth 97: 701-703, 2006
5-28. Acceptable/Low Risk Procedures in FOP
In FOP, it is important to avoid soft-tissue trauma as it is likely to induce flare-ups and rapidly progressive HO, with resultant permanent loss of motion in the affected area (Kitterman et al., 2005). All invasive procedures in an FOP patient carry risk. Although patients with FOP must occasionally undergo medical procedures, the range of acceptable/low risk procedures remains undefined, so performing any procedure must be judged considering the potential risks and the benefits.
Injection and Venipuncture
Intramuscular injections and immunizations should be avoided because they can lead to permanent loss of movement (Kitterman et al., 2005). Intradermal or subcutaneous injections are considered acceptable. No immunizations should be given while the patient is experiencing an active flare-up or within 2 weeks of a flareup. For coronavirus vaccinations, please see section on COVID-19.
Local anesthesia can be risky but may be acceptable for dermatologic procedures such as mole removal, if needed. For other indications, please consult directly with an FOP expert. For dental use, please see section on dental anesthesia.
Blood sampling can be safely performed from subcutaneous veins. Peripheral intravenous catheterization is also considered safe, if atraumatic. Placing femoral vein and radial artery catheters without any complications is reported (Liu et al., 2014), but is considered very high risk. Arterial catheterization will cause HO and is routinely forbidden unless critical for patient care. PICC lines have been used in patients with FOP but have an increased risk of deep vein thrombosis (DVT; due to decreased mobility or anatomic restriction). Also, PICC lines may have an increased risk of HO at the insertion site.
Surgery and Other Invasive Procedures
In many children with FOP, tender soft-tissue masses initially develop mainly on the head, neck, or back (Kitterman et al., 2005). Without the previous diagnosis of FOP, clinicians tend to suspect neoplasm, leading to high rates of biopsy and/or resection surgery, with subsequent HO (Kitterman et al., 2005, Zaghloul et al., 2014). Orthopedic surgeries to remove HO or to correct deformities in the extremities or trunk have been reported, but most of them lead to HO and worsening of motion/deformity, even though medications to prevent inflammation and ossification were used perioperatively.
Reports on other invasive procedures are limited and the safety is not established. Lumbar puncture as a routine work-up for an intractable fever in an infant led to flare-up (Zaghloul et al., 2014). Emergent laparotomy for peritonitis evoked no HO (Okamoto et al., 2020). Physiotherapy is reported to induce permanent complications (Kitterman et al., 2005). However, the range of acceptable physiotherapy procedures has not been established. Passive range of motion exercises to gain additional range of joint motion should be avoided, but gentle active exercise may be permitted.
References
Kitterman JA. Kantanie S, Rocke DM, Kaplan FS: Iatrogenic harm caused by diagnostic errors in fibrodysplasia ossificans progressiva. Pediatrics 116: e654-e661, 2005
Liu JX, Hu R, Sun Y, Jiang H: General anesthesia in fibrodysplasia ossificans progressiva: a case report and clinical review. Int J Clin Exp Med 7: 1474-1479, 2014
Okamoto N, Tazaki T, Shintakuya R, Hirano T, Sasaki M, Takahashi S, Nakamitsu A. Fibrodysplasia ossificans progressiva with two emergency laparotomies: A case report. Int J Surg Case Rep 73: 196-198, 2020
Zaghloul KA, Heuer GG, Guttenberg MD, Shore EM, Kaplan FS, Storm PB: Lumbar puncture and surgical intervention in a child with undiagnosed fibrodysplasia ossificans progressiva. J Neurosurg Pediatr 1: 91- 94, 2008
5-29. Hearing Impairment in FOP
Hearing impairment is a common feature of FOP and occurs in approximately 50 percent of patients. The onset is usually in childhood and may be slowly progressive. Hearing loss is usually conductive in nature and may be due to middle ear ossification, but in some patients, the hearing impairment is neurologic in nature. Children with FOP should generally have audiology evaluations every other year; more often, if necessary. Hearing aids are often helpful and can diminish developmental problems due to hearing loss (Levy et al., 1999). Acute hearing loss is not usually associated with FOP and should prompt evaluation for other causes.
References
Levy CE, Lash AT, Janoff HB, Kaplan FS. Conductive hearing loss in individuals with fibrodysplasia ossificans progressive. Am J Audiol 8: 29-33, 1999
5-30. Gastrointestinal Issues in FOP
According to baseline data collected by the IFOPA Registry (Mantick et al, 2018) almost 28% of all patients report at least one health issue related to the stomach and digestion. Abdominal pain was the most common complaint, accounting for about 18% of all participants, followed by symptoms of gastrointestinal reflux and nausea. Abdominal pain was highest in females over the age of 17. Difficulty swallowing was reported in up to 19% of males age 18 years or older. Loss of appetite was most common among adult females. Rectal complaints, including constipation, was present in about 10% of adult patients, more than twice as much compared to those under the age of 18. Severe diarrhea was a complaint in approximately 5% of patients. Trouble digesting food and complaints of intermittent vomiting represented about 7.5% and 9% of all registry participants, respectively. Overall, the subgroup most affected by gastrointestinal issues is adult females, and the subgroup least affected is females under the age of 18.
Functional gastrointestinal disorders, including dyspepsia and irritable bowel syndrome, have been associated with low-grade mucosal inflammation dominated by mast cells, eosinophils and intraepithelial lymphocytes (Wouters et al., 2016), suggesting a possible link between local immune-mediated phenomena and some gastrointestinal complaints in FOP. Anecdotally, the use of mast cell inhibitors for these complaints has provided relief of symptoms in selected, otherwise unresponsible cases.
It is unclear at the present time if the prevalence of these complaints is greater than those in the general population, demographically matched, or if the etiologies of these complaints differ based on the diagnosis of FOP.
Ahn et al reported a patient with FOP who had chronic abdominal pain, decreased appetite, biliary vomiting, and weight loss leading to the diagnosis of superior mesenteric artery (SMA) syndrome (Ahn et al., 2023). SMA syndrome is a rare condition in which the third part of the duodenum is compressed by the aorta and superior mesenteric artery due to a paucity of peri-mesenteric fat, resulting in duodenal obstruction. Clinical suspicion of SMA syndrome should be considered in any FOP patient who has persistent weight loss, gastrointestinal symptoms, and biliary vomiting. In FOP, SMA syndrome is often amplified by scoliosis, immobility and ankylosis of the jaw leading to poor caloric intake. Although SMA is difficult to diagnose due to its infrequency and diverse symptoms, it is important to consider the diagnosis of SMA syndrome in patients with FOP. Abdominal CT scan can be diagnostic (Ahn et al., 2023). Treatment of SMA syndrome in FOP should first be conservative. A nasogastric tube can be inserted for decompression of the duodenum or stomach. Weight gain through intravenous nutrition or a nasal-jejunal tube should be considered. If possible, the patient can be placed in a prone or left lateral position to decrease pressure on the duodenum. Invasive procedures can potentially trigger flare-ups, so they should only be performed when absolutely necessary (Ahn et al., 2023).
References
Ahn TY, Han JB, Bae JY, Woo SH. Superior mesenteric artery syndrome in a patient with fibrodysplasia ossificans progressiva. Bone Rep 2023 Jul 20;19:101702
Mantick N, Bachman E, Baujat G, Brown M, Collins O, De Cunto C, Delai P, Eekhoff M, Zum Felde R, Grogan DR, Haga N, Hsiao E, Kantanie S, Kaplan F, Keen R, Milosevic J, Morhart R, Pignolo R, Qian X, di Rocco M, Scott C, Sherman A, Wallace M, Williams N, Zhang K, Bogard B. The FOP connection registry: Design of an international patient-sponsored registry for Fibrodysplasia Ossificans Progressiva. Bone 109: 285-290, 2018
Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut 65: 155-168, 2016
5-31. Kidney Stones & FOP
Clinical observations prompted a worldwide survey of patient-members of the International Fibrodysplasia Ossificans Progressiva Association (IFOPA) on the disease burden of kidney stones. The survey examined risk factors for the development of kidney stones in FOP patients and provided a basis for prevention of stones in this already devastating disease (Gupta et al., 2018).
Although geographical variation exists, patients with FOP have approximately a two-fold higher prevalence of kidney stones than the general population. Immobilization coupled with increased bone turnover is a significant risk factor in the development of kidney stones in this population. There has been no comprehensive study of stone composition in FOP patients.
A low-fiber diet was the only dietary factor in this study to significantly increase the risk of developing kidney stones in this population, although deficient water intake and excess animal protein intake were associated with the condition. FOP patients with a history of urinary tract infections are at increased risk for developing kidney stones. Extracorporeal shock wave lithotripsy, uteroscopic stone removal, percutaneous nephrolithotomy, and laser lithotripsy have all been used as treatment modalities, but there are no long-term data to evaluate the safety or efficacy of one treatment over another.
Ideally, recommendations to prevent kidney stones are made. This becomes increasingly important as FOP patients become progressively more immobilized. Patients should (Gupta et al., 2018):
Drink sufficient water to keep the urine volume above two liters daily.
Substitute whole wheat bread for white bread and eat natural fiber cereals.
Limit their intake of Vitamin C and oxalate-rich foods.
Refrain from adding salt to their food.
Obtain the recommended dietary allowance (RDA) of calcium, which would be 1,000 mg of elemental calcium daily for adults, age 18-50 years old.
References
Gupta RR, Delai PLR, Glaser DL, Rocke DM, Al Mukaddam M, Pignolo RJ, Kaplan FS. Prevalence and risk factors for kidney stones in fibrodysplasia ossificans progressiva. Bone 109: 120-123, 2018
5-32. Rehabilitation Issues in FOP
As degenerative joint disease advances and heterotopic bone accumulates in FOP, range of motion is progressively lost, leading to near complete immobility (Pignolo et al., 2016; Kaplan et al., 2017; Kaplan et al., 2018; Pignolo et al., 2018). Until this process can be halted or reversed, the focus of rehabilitation approaches should be on preserving and promoting independence and enabling activity and participation in daily living. Occupational therapy and vocational education consultations may be extremely useful. Although protected active range of motion such as warm water hydrotherapy is encouraged, passive range of motion (motion performed by someone other than the patient in other words, a movement of a joint carried out by an operator without aid of the patient's muscles) must be avoided, as it risks exacerbation of FOP with further loss of function and pain. Despite widespread HO and progressive disability, most individuals with FOP lead productive and fulfilling lives (Levy et al., 1999; Levy et al., 2005).
Many of the limitations exacerbated by disease progression can be ameliorated with thoughtful rehabilitation.
Occupational Therapy Interventions: Dressing may be enabled with pullover shirts and blouses, elastic waistbands, Velcro closures, sock donners (devices where the sock is placed over a cuff attached to a cord), elastic shoelaces, and long handled shoehorns and reachers. Raised toilet seats, custom-angled commodes, bedside urinals (shaped for men or women), and bidets all enable toileting. Widened doorways, tub and bath benches (which may need to be customized to fit the individual) and grab bars increase bathroom safety and accessibility. Long-handled sponges, combs, or modified reachers, electric toothbrushes, water pics, and suction devices help insure cleanliness and personal hygiene.
Strategically placed stools and elevated platforms, long-handled eating utensils and straws, help at the dinner table. Meal preparation may be facilitated by electrical can and jar openers, cutting boards with spikes to hold food while it is prepared or cut, and rotating shelves (Lazy Susans). For individuals with limited ability to masticate, food may be ground-up or pureed.
Depending on the stage of disease progression, canes, walkers, crutches, and/or custom shoes may be essential aids to ambulation. The assistance of thoughtful orthotists to fabricate adaptive shoes and inserts should be considered. For more severe limitations, power wheelchairs may be necessary.
Considerations for power wheelchairs include customized seating, power seat elevation and depression, anterior and posterior tilt and recline functions. Lap trays with mounts for laptop computers allow participation in work and school.
Vocational and Educational Issues: Because even minor trauma can trigger disabling HO, it is sensible to encourage intellectual pursuits and computer skills as alternatives to physical activity. The Individuals with Disabilities Education Improvement Act of 2004 (IDEA 2004) is a law in the United States that mandates equity, accountability and excellence in education for children with disabilities. This requires public school systems to provide each disabled child with an individualized educational plan, and an education in the least restrictive environment. Children are entitled to occupational, physical and speech therapy as well as classroom aides, if indicated. Each state is required to offer some sort of vocational rehabilitation to help people with disabilities enter or remain in the work force.
Transportation and Home Modification: Vans can be customized to accept an FOP power chair. Ramps and lifts can be installed, roofs can be raised, floors can be lowered, new controls and motors can be installed to allow the van to “kneel”, lowering ground clearance to ease ascent into a van.
Home modifications include elimination or minimization of indoor steps, installation of grab bars as well as exterior and interior ramps, widened hallways, accessible bathrooms and kitchens.
Environmental control units (which may be integrated as smart phone applications) operate appliances, doors, and televisions remotely. To facilitate sleep, there are tilt table beds that rotate from vertical to horizontal, specialized mattresses and overlays to redistribute pressure to provide comfort and protect skin integrity.
Sexuality and reproduction: Physical acts of sexual intimacy require tact and thoughtfulness. Pillows and bolsters may be necessary to support unusual and inflexible postures. Genetic counseling and discussion of contraception are warranted for the sexually active or those who are considering such activity, especially if they or a partner are in a clinical trial and/or on an investigational agent.
Recreational therapy: Recreational therapy may be helpful in making meaningful use of leisure time. Psychologists, social workers and other mental health counselors can help affected individuals and family members with adjustment to the limitations and inconveniences imposed by FOP. Creative arts therapies (art therapy, music therapy, dance/movement therapy, drama therapy, poetry therapy) use arts-based methods and creative processes for the purpose of ameliorating disability and illness and optimizing health and wellness.
Many of these rehabilitation treatment approaches can be delivered, at least in part, directly to the home via telehealth.
Aquatic therapy (warm water hydrotherapy) allows individuals to perform active range of motion, cardiopulmonary, and resistive exercise in a safe, low impact environment. Warm water can facilitate pain relief. Modified lifts, elevators or ramps may be necessary for pool entry and exit.
Iontophoresis involves the introduction of topically applied physiologically active ions (acetic acid, steroids) through the epidermis using continuous direct current. Anecdotal reports suggest that acetic acid iontophoresis may help restore some lost temporomandibular joint range of motion in FOP.
For questions on rehabilitation for FOP patients, please contact:
Charles Levy, MD
Adjunct Associate Professor, Department of Physical Therapy Research Scholar, Center for Arts in Medicine
University of Florida 426 SW 43rd Terrace
Gainesville, Florida 32607, USA Email: levyce@aol.com
References
Kaplan FS, Al Mukaddam M, Pignolo RJ. A cumulative analogue joint involvement scale for fibrodysplasia ossificans progressiva (FOP). Bone 109: 123-128, 2018
Kaplan FS, Al Mukaddam M, Pignolo RJ. Longitudinal patient-reported mobility assessment in fibrodysplasia ossificans progressiva (FOP). Bone 109: 150-161, 2018
Levy CE, Berner TF, Bendixen R. Rehabilitation for individuals with fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab 3: 251-256, 2005
Levy CE, Berner TF, Sandhu PS, McCarty B, Denniston MA. Mobility challenges and solutions for fibrodysplasia ossificans progressiva. Arch Phys Med Rehab 80: 1349-1353, 1999
Pignolo RJ, Bedford-Gay C, Liljesthröm M, Durbin-Johnson BP, Shore EM, Rocke DM, Kaplan FS. The natural history of flare-ups in fibrodysplasia ossificans progressiva: a comprehensive global assessment. J Bone Miner Res 31: 650-656, 2016
Pignolo RJ, Durbin-Johnson BP, Rocke DM, Kaplan FS. Joint -specific risk of impaired function in fibrodysplasia ossificans progressiva (FOP). Bone 109: 124-133, 2018
5-33. Aids, Assistive Devices, and Adaptations (AADAs)
Effects on global health by use of AADAs
Aids, assistive devices and adaptations (AADAs) are frequently used by individuals with FOP. In a study on 299 FOP patients from 54 countries, based on data from the International FOP Association (IFOPA) Global Registry, there was an increase in the use of AADAs by age groups, but no difference on the basis of gender for all participants (Pignolo et al., 2020). The median number of AADAs was 2.0 for the 0–<9 years age group, 7.5 for the 9–15 years age group, and 13.0 for the>15 years age group. The most commonly used AADAs, each by >40% of all participants, were bathing attendants, straws for drinking, reaching sticks, and memory foam bed mattresses. The use of some AADAs were associated with improvement in global health scores, including customized motorized wheelchairs, drinking draws, portable urinals, pill crushers, roll-in showers, portable commodes, airflow mattresses, ramps for home entry, automatic lights, customized counters, durable flooring, remote control entry, adaptive keyboards, adaptive chairs, special electronics, adapted vehicles for motorized wheelchairs, wide doorways for wheelchair access, voice activated computer software, typing sticks, and onscreen keyboards not normally part of devices.
Anticipatory guidance for AADAs
It is difficult to predict when a given individual with FOP will first experience impairment in activities of daily living that can be mitigated by AADAs. However, based on use by at least 10% of IFOPA Global Registry participants, it is possible to give anticipatory guidance on the type of ADAAs that are likely to be required over the natural course of the disease (Table). Evaluation of AADA use among age groups provides several high-level recommendations: (1) Several AADAs are valuable across all age groups, including bathing attendants, part-time home personal care attendants, straws for drinking, reaching sticks, customized clothing, memory foam bed mattresses and pillows, floor level thresholds on all door-ways, and customized furniture; (2) Among personal care tools and aids, dressing sticks and hearing aids become very helpful or required, respectively, beginning at 9 years of age; (3) Many bathroom aids and devices are frequently employed by age 9; (4) Home adaptations are generally required after the age of 15; (5) Occupational therapy and devices used to preserve pulmonary function are useful over the entire life span.
Practical experience suggests that patients and families seek AADAs when a physical limitation demands remediation and support rather than in anticipation of such limitation. This reactionary behavior causes excessive stress in a time when the patient is already experiencing extreme challenges of overcoming new limitations from the progression of the disease. This is often the result of social support systems that, although well intentioned, enable dependent behaviors rather than empower independent activities through the early utilization of AADAs. An approach to reduce enabling behaviors and encourage patient independence through AADA use is to introduce the tool or adaptation prior to its need, in a setting where it is visible and promoted by the entire family. For example, providing a child (who has not yet lost mobility) with a reaching device and taking turns with them picking out socks from the laundry basket. In this way there is a greater chance for acceptance and use of the tool or adaptation because time is given for the emotional and physical adjustment period before the need is actually realized. The window of opportunity for introduction of an AADA must be considered in light of the usual sequence of events—from pre- limitation, to limitation, initial AADA resistance, and slow acceptance. This anticipatory planning has the potential to provide patients with increased opportunities for success using AADAs leading to improved coping skills, greater resiliency, increased emotional intelligence and overall social-emotional well-being as the disease progresses.
Most commonly recommended AADAs
The following have been identified as some of the most commonly used AADAs based on consultations and inquiries made by medical support staff and FOP community members, respectively, to the IFOPA. For AADA consultations and personalized support go to www.ifopa.org/ability_toolbox_program.
Most common Early Childhood AADAs:
Protective headgear (helmets, impact-reducing headbands) to protect the head and face during falls
Gait trainers to assist with balance when learning to walk
Adaptive strollers that provide extra cushion and support for community transportation
Floor padding and bumpers for safe floor play
Sink faucet extenders to shorten the reaching distance when washing hands
“Nosey” cups – cups with a section of rim removed to make space for the nose so that head extension is not necessary for drinking
Bendable foam tubing grips to improve grasp and holding angle of flatware, toothbrushes, and/or pencils
Most common School-Aged and Young Adult AADAs:
Dressing sticks and long-reach shoehorns to assist with dressing
Adaptive clothing with additional zippers, Velcro, or magnetic closures
Adaptive desk chairs that swivel to allow full visual field despite reduced neck mobility
Adaptive desk with adjustable height to accommodate adaptive seating or wheelchairs
Table-top slant boards to support easier viewing and writing angles on assignments/documents
Keyboard typing aids
“Pick Me” stick or visual aid to extend in lieu of child raising hand to be called upon in class
Bean bag chairs for more comfortable seating when on the floor
Individualized Educational Plans to allow for necessary accommodations in school (flexible seating, longer test durations, early release when transitioning between classes to avoid crowded hallways and risk of falls, alternate activities during Physical Education classes, para-professional assistance)
Adaptive bicycles and recreational gear
Toilet wiping aids
Hair washing tools
Emergency ID bracelets to inform First Responders, school staff, and/or employers of FOP dx and contraindications
Most common Adult AADAs:
Extended eating utensils and adaptive dishware (high-sided plates for easier scooping, plates with spikes to hold food in place while cutting)
Bathing and hygiene tools, adaptive combs/brushes, adaptive shavers, adapted cosmetic make-up applicators
Adapted toilets (Bidet toilet seats, toilet risers, grab bars or side rails for support, portable commodes and urinals)
Shower chairs and zero-entry (“roll-in”) showers
Canes, Lofstrand crutches or rolling walkers to assist with balance when walking
Motorized scooters to assist with mobility for extended distances
Custom motorized wheelchairs for non-ambulators and wheelchair accessories (i.e. trays, mounts for cell phones and tablets, cup holders, custom-placed joystick controls based on upper extremity positioning)
Cushions and seating solutions to prevent pressure sores
Wheelchair access ramps, both permanent and portable, allowing access into home environments and public spaces
Accessible vans for transporting wheelchair users
Individuals with FOP tend to use AADAs available in the marketplace as well as homemade tools they have personally crafted for their individual needs. Knowledge and accessibility of AADAs vary greatly across the globe; therefore, encouraging patients to use creativity and a problem-solving mindset to generate solutions to their mobility challenges is vital to their success with independence. Everyday items such as coat hangers, duct tape, dowel rods or sticks, and pipe or tubing can be used to construct a tool that helps a patient reach, scratch, push and pull in order to complete a task, demonstrating that expensive AADAs found in the marketplace can be simulated and are often not necessary. While referring patients to the IFOPA for support with AADAs is encouraged, it is also recommended to seek input from the national FOP organizations for country-specific resources as availability of AADAs, cost, healthcare assistance, and importing restrictions vary greatly by geographic location.
Description of online resources for AADAs
In addition to finding AADAs through internet searches, adaptive equipment catalogs and recommendations from allied health professionals, there is a collection of online resources for AADAs available on the IFOPA website at https://guidebook.ifopa.org/. The AADAs in the Online Guidebook can be downloaded and printed for those without web access, and the entire resource has a translation feature using Google Translate. This resource is an ever-changing library of AADAs reflecting the current trends of tool use in the FOP community throughout the world and is updated as new tools are discovered and submitted to the IFOPA.
For individualized support beyond AADAs listed in the Online Guidebook, medical professionals are encouraged to refer patients to:
Family support programs (www.ifopa.org/support_groups)
IFOPA Family Services Coordinator and Ability Toolbox program (www.ifopa.org/ability_toolbox_program)
Harold & Elaine Kaplan Quality of LIFE Award program for financial support for AADAs (www.ifopa.org/quality_of_life_award)
Medical professionals may also request a sample demonstration kit of commonly used AADAs for their office to share with families during office visits.
Doctor’s Medical Tool Kit of sample AADAs (www.ifopa.org/toolkits_for_healthcare_providers)
References
Pignolo RJ, Cheung K, Kile S, Fitzpatrick MA, DeCunto C, Al Mukaddam M, Hsiao EC, Baujat G, Delai P, Eekhoff EMW, Di Rocco M, Grunwald Z, Haga N, Keen R, Levi B, Morhart R, Scott C, Sherman A, Zhang K, Kaplan FS. Self-reported baseline phenotypes from the International Fibrodysplasia Ossificans Progressiva (FOP) Association Global Registry. Bone 2020;134:115274
5-34. Women’s Health in FOP
We have observed that women with FOP can have various menstrual abnormalities ranging from primary amenorrhea (i.e., lack of initiation of menstrual cycle after the age of 15 years), secondary amenorrhea (i.e., absent menstrual for 3-6 months after the onset of menarche), irregular menstrual cycles and uterine fibroids leading to heavy menstrual cycles.
We recommend evaluation for all women with primary and secondary amenorrhea and consider hormonal replacement therapy with estrogen (+/- progesterone) for bone health. Oral or transdermal patches have been used safely in women with FOP for hormonal replacement. Intra-uterine devices (IUDs) should be avoided. Intramuscular injections or implanted contraceptives are absolutely contraindicated.
For women with heavy menstrual cycles and concern for hygiene, oral contraceptive can be used to decrease or stop the menstrual cycle. Hormonal replacement can be associated with increased risk of blood clots; risks and benefits of starting oral contraception needs to be discussed with their local providers. No data exist to suggest that oral contraceptive/hormone replacement increase the risk of flare-ups.
Many adult women with FOP have reported a history of uterine fibroids (leiomyoma); the exact prevalence in FOP is yet to be fully determined. Based on the IFOPA registry from September 2023, 9/19 (47%) of the female respondents (age range 19-56 years) reported history of uterine fibroids. Uterine fibroids are the most common benign pelvic tumor in females. Reported prevalence is extremely variable depending on the age and race of the cohort as well as methods for identifying uterine fibroid (symptoms, ultrasound, or pathology). A retrospective study reviewing charts of 277,821 women age range 18–65 years followed at Kaiser Permanente noted an overall prevalence of nearly 10% (Yu et al., 2018). In a surgical study, the prevalence of uterine fibroid was noted in 77% of surgically removed uteri (Cramer and Patel, 1990).
Management of uterine fibroid will depend on the symptoms. Hormonal medications (oral contraception, GnRH agonist) can be used to decrease bleeding. NSAIDs (example Ibuprofen) can be used to decrease pain and menstrual bleeding. Tranexamic acid can decrease bleeding.
Invasive and surgical procedures should be avoided in patients who have FOP, if possible. If medically necessary, hysterectomies can be performed under the guidance of a multi-disciplinary team (Ho et al., 2021).
Oral contraceptives are not contraindicated in FOP. However, some patients have reported changes in their flare activity when on cyclic oral contraceptives. Formats such as progestin-only pills or versions with fewer endometrial shedding cycles (such as Seasonale Contraceptive Oral, with 4 periods/year) may be useful to consider.
References
Ho M, Park BY, Rosenblum NG, Al Mukaddam M, Kaplan FS, Kucherov V, Hubosky SG, Kane G, Desai V, Kramer MR, Ku BS, Schwenk ES, Baratta JL, Harshavardhana D, Grunwald Z. Surgical and Radiological Management of Complicated Uterine Leiomyoma Aided by 3D Models in a Patient with Fibrodysplasia Ossificans Progressiva. Am J Case Rep 2021 Jun 10;22:e931614.
Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol 94: 435-438, 1990
Yu O, Scholes D, Schulze-Rath R, Grafton J, Hansen K, Reed SD. A US population-based study of uterine fibroid diagnosis incidence, trends, and prevalence: 2005 through 2014. Am J Obstet Gynecol 2018 Dec;219(6):591.e1-591.e8.
5-35. Pregnancy Issues in FOP
The decision to have a child is one of the most important and serious life decisions an individual or couple can make. Because FOP is an inherited disease, anyone (man or woman) with FOP will have similar concerns about passing the FOP mutation to his/her child. If a parent has FOP, the chance that the child will have FOP is fifty percent. Women, specifically, have additional matters to consider. In addition to the usual risks that any woman might encounter during pregnancy, a woman with FOP has additional concerns that must be carefully considered (Davidson et al., 1985; Fox et al., 1987; Thornton et al., 1987; Muglu et al., 2012; Forrest et al., 2022).
Pregnancy is a rare event in FOP; however, it is possible for a woman with FOP to carry a child (Muglu et al., 2012). However, pregnancy and childbirth have substantial life-threatening risks to both the mother and child and present unique management challenges. If pregnancy is contemplated by a woman with FOP, pre-pregnancy counseling is mandatory.
FOP results in breathing difficulties during the latter part of pregnancy because of restrictive chest wall disease due to developmental anomalies in the costovertebral joints and progressive HO in the chest muscles and abdominal wall. Careful monitoring including respiratory function tests may be required during the antepartum and intrapartum course to establish any requirement for respiratory support.
A pregnancy, even with an unaffected fetus, poses substantial risks to a mother with FOP. Although data are scant, one of the initial risks of pregnancy associated with FOP is a risk of miscarriage or substantial risk of premature birth following spontaneous preterm labor and may be secondary to fetal distress encountered during the later stages of pregnancy. Steroid administration for fetal lung maturity is imperative if delivery is anticipated before 36 weeks of gestation (Muglu et al., 2012).
Another substantial risk is thromboembolism, which is exacerbated by severe immobility of FOP in addition to the hyper-coagulable state of pregnancy. The added constraint of lower limb edema that invariably occurs in the last trimester of pregnancy further increases the risk of this life-threatening complication and warrants consideration of the use of prophylactic low-molecular-weight heparin throughout the duration of pregnancy (Muglu et al., 2012).
In addition to substantial risk to a mother with FOP, pregnancy also poses substantially increased risks to the unborn child including the risk of FOP (50%), prematurity, fetal distress and the risk of complications from requisite general anesthesia. One of the specific risks to the mother associated with FOP is a flare-up during pregnancy. The chronic use of high-dose glucocorticoids and non-steroidal anti-inflammatory medications has potential embryonic and fetal toxicity, and their use should be avoided, when possible.
The management of miscarriage, delivery and antenatal care poses substantial difficulties because of the specific risks associated with FOP. Vaginal delivery is perilous in a woman with FOP due to severe pelvic deformity as well as fusion of the lumbar spine, hip joints and sacroiliac joints. Delivery by caesarean section is the only relatively safe mode of delivery after the age of viability is achieved (Muglu et al., 2012). Uterine bleeding needing transfusion and/or hysterectomy after caesarean have been reported (Forrest et al, 2022).
In addition to the physical problems associated with delivery, the choice of anesthesia becomes a challenge due to technical difficulties with both regional and general anesthesia. Regional anesthesia is technically difficult due to pre-existing heterotopic bone and the danger of precipitating new episodes of HO following an epidural block. Similar problems, including ossification in tracheal rings and the danger of overstretching the jaw, may cause difficult intubation during general anesthesia. Also, it is impossible to extend the neck in young adults with FOP due to orthotopic ankylosis of the cervical paravertebral joints that ossify in childhood, even before the appearance of HO. The use of awake fiberoptic nasotracheal intubation was found to be the only safe option. At delivery, there should be a team skilled in resuscitation of high-risk infants (Muglu et al., 2012; Kilmartin et al., 2014).
Many reports describe exacerbation of FOP following surgical procedures. However, in other cases (five surgical operations including two hysterectomies), no HO formed in the abdominal wall. The use of prednisone or intravenous equivalent at the time of elective surgery and three days following surgery is recommended if there are no other contraindications. The rational use of corticosteroids early in the course of an FOP flare-up is based primarily on its potent anti-inflammatory effects and on emerging knowledge of the importance of inflammatory triggers in FOP flare-ups (Muglu et al., 2012).
In a retrospective case series, Forrest and colleagues describe three women who delivered preterm infants at one institution during a 10-year time span (Forrest et al., 2022). These cases posed unique anesthetic and obstetric technical challenges, particularly when securing the airway and performing cesarean delivery.
Importantly, each patient received perioperative glucocorticoids for prevention of further heterotopic ossification and required multidisciplinary management for optimal outcomes.
Although in vitro fertilization, pre-implantation genetic testing, embryo selection and surrogate motherhood are theoretically possible now after the gene discovery, that sequence has not been reported for FOP. Prenatal genetic diagnosis could potentially be used to exclude FOP (Du et al., 2010).
In summary, although pregnancy in women with FOP is possible, FOP poses major life-threatening risks to mother and child as well as life altering consequences to the entire family if a child is born with this condition. In addition, pregnancy is absolutely contraindicated during participation in a clinical trial.
Pregnancy in FOP should never be undertaken without serious consideration and family planning. Unwanted pregnancies should be assiduously avoided. Independent genetic counseling is available, if desired. Should a pregnancy occur, guidance and care at a high-risk pregnancy center are imperative.
References
Davidson BN, Bowerman RA, La Ferla JJ. Myositis ossificans progressiva and pregnancy. A therapeutic dilemma. J Reprod Med 30: 945-947, 1985
Du J, Huang LL, Tan YQ, Cheng DH, Li SF, Li LY, Lu GX. Mutation analysis and prenatal exclusion of fibrodysplasia ossificans progressiva in a Chinese fetus. Genet Test Mol Biomarkers 2010 Jan 10
Forrest AD, Vuncannon DM, Ellis JE, Grunwald Z, Kaplan FS. Fibrodysplasia Ossificans Progressiva and Pregnancy: A Case Series and Review of the Literature. Case Rep Obstet Gynecol 2022 Sep 16;2022:9857766
Fox S, Khoury A, Mootabar H, Greenwald EF. Myositis ossificans progressiva and pregnancy. Obstet Gynecol 69 (Pt 2): 453-455, 1987
Kilmartin E, Grunwald Z, Kaplan FS, Nussbaum BL. General anesthesia for dental procedures in patients with fibrodysplasia ossificans progressiva: a review of 42 cases in 30 patients. Anesth Analg 118: 298-301, 2014
Muglu JA, Garg A, Pandiarajan T, Shore EM, Kaplan FS, Uchil D, Dickson MJ. Pregnancy in fibrodysplasia ossificans progressiva. Obstet Med 5: 35-38, 2012
Thornton YS, Birnbaum SJ, Lebowitz, N. A viable pregnancy in a patient with myositis ossificans progressiva. Am J Obstet Gynecol 156: 577-578, 1987
5-36. FOP Variants
[Adapted from “FOP Variants: What Are They, Who Has Them & What Do They Mean for You? By Frederick S. Kaplan, M.D. and Eileen M. Shore, Ph.D.”]
When we started seeing FOP patients, nearly 35 years ago, it quickly became obvious that everyone shared two features: malformed big toes and progressive HO. These were clearly two characteristic clinical features, and they defined classic FOP.
As we saw more patients, we recognized variability in the toe malformation that individuals had, as well as differences in the rate of progression of HO. For example, some had short, bent big toes; others had short, straight big toes; others still had long big toes, and some were of normal length. But everyone had a toe malformation – most commonly characterized by a missing or malformed joint in the big toe that was obvious on physical examination, radiographs or both. Likewise, we noted variability in the rate of progression of the FOP HO – some progressed very rapidly while others progressed very slowly, and still others progressed at a more even pace. Much like other traits in any population, FOP showed a natural variation that defined the limits of the norm.
Occasionally though, we saw someone who had a feature of FOP that was WAY outside of the “normal” range – even for those with FOP. This defining feature most often involves the big toes. Among these individuals, we began to recognize two groups: One group had nearly normal or completely normal-looking big toes. The other group had extremely severe toe malformations that involved other digits in the feet and the hands. In the more “severe” group, we observed additional developmental abnormalities in other organ systems. We refer to these two groups of outliers as “FOP variants” – some mild, some severe.
Approximately 97% of individuals that we have seen with FOP HO had “classic FOP”, and approximately 3% of individuals were “FOP variants.” About half the patients with FOP variants (1.5%) had a mild clinical variant and about half the patients (1.5%) had a severe variant. Again, these observations were based on clinical evaluation and preceded the discovery of the FOP gene.
After we discovered the FOP gene, we examined the DNA sequence of the FOP gene in all patients who we had seen. Remarkably, nearly every single patient who was diagnosed as having “classic FOP” – regardless of where they were on the spectrum of disease severity – had the same exact heterozygous missense activating FOP mutation: [ACVR1c.617G>A; p.R206H].
As remarkable, every single patient who we had identified clinically as an “FOP variant” had a different heterozygous missense activating mutation in the ACVR1 gene.
In other words, those with “classic FOP” as the clinical diagnosis had the same “classic mutation” in the FOP gene [ACVR1c.617G>A; p.R206H], while everyone clinically diagnosed as an “FOP variant” had a “variant mutation” in the FOP gene.
While many will ask, “Is the HO less severe in the less severe variants and more severe in the more severe variants?” The answer is: “Sometimes, but not necessarily.” Some of the patients with mild toe variants have a later onset and a milder course of HO and some of the patients with severe toe variants have an earlier onset and more severe course of HO. But there is wide variability – just as there is in the onset and severity of HO in the patients with “classic FOP,” even among identical twins with classic FOP. The most important defining clinical feature of the “FOP variants” is the malformation of the big toes – either far less severe or far more severe than the patients with “classic FOP”.
Although the clinical assessment is extremely important in assigning a clinical status of “classic FOP” vs. “FOP variant”, the only way to ascertain the exact type of FOP at a molecular level is by genetic testing and DNA sequence analysis of the FOP gene. To be clear, the absolute defining factor in whether someone has “classic FOP” or an “FOP variant” is the exact genetic sequence of the ACVR1 (FOP) gene. If someone has the commonly shared ACVR1c.617G>A; p.R206H mutation, then they have “classic FOP”. If they have a variant genetic mutation in the ACVR1 gene, then they have an “FOP variant”. So far, there are approximately 20 identified variants in the FOP gene.
The evaluation of the FOP gene (ACVR1) by DNA sequencing can be conducted in a genetics laboratory through DNA obtained from a blood sample. The analysis can be arranged by your physician.
Genotyping is required for enrollment into all clinical studies and is important for proper clinical and genetic counseling.
Keep in mind that FOP variants are much rarer than classic FOP. Some of the ACVR1 variants have so far been found in only one or two affected individuals in the world, so it is difficult to make predictions about the course of FOP over time. With other variants, there may be a few affected individuals in the world, so we know a little bit more about the course that FOP variant may take over time.
So, what does this all mean for someone who has an FOP variant?
First, we have less knowledge and therefore less certainty about the FOP variants than we do about classic FOP; but we and other scientists are beginning to learn more about how ACVR1 variant mutations affect cell functions and how they are similar to and different from the classic ACVR1 mutation (Haupt et al., 2018; Mucha et al., 2018; Allen et al., 2020). The exact location and characteristic of the mutation in the ACVR1 gene (blueprint for the ACVR1 protein; classic vs. variant) informs the structural biologists with whom we work and collaborate to better understand the damaged workings of ACVR1 in FOP. That insight is critical to developing structural models and approaches to inactivating the damaged and overactive switch that leads to disabling HO in all forms of FOP.
Second, regardless whether someone has “classic FOP” or an “FOP variant”, all have over-activity of the bone forming BMP signaling pathway and thus the tendency to form heterotopic bone.
Third, whether someone has “classic FOP” or an “FOP variant”, the process by which they form heterotopic bone after birth is the same.
Fourth, the general precautions for FOP are the same for patients with classic FOP and FOP variants.
Fifth, the symptomatic management of flare-ups is the same for patients with classic FOP and FOP variants.
Sixth, some of the approaches to develop medications for FOP are mutation-specific while others target the broad process of HO common to both.
Seventh, approaches to specifically block the overactive ACVR1 receptor (encoded by the FOP gene) should be applicable to FOP variants as well as classic FOP.
Eighth, new clinical trials will likely be limited at first to patients who have classic FOP – and then later, if applicable, to those with FOP variants – based primarily on regulatory requirements.
Ninth, every measure and pressure are being exerted to open-up applicable clinical trials to patients with FOP variants as quickly and humanly possible. This is being done right now.
Tenth, and finally, all patients with FOP – classic and variant FOP – are part of the small but powerful worldwide FOP community. There is a common thread that unites everyone with FOP. All with FOP must stay together, speak with one voice, and learn from each other. Knowledge will lead to better treatments and a cure for all of those with FOP regardless of whether one has “classic FOP” or an “FOP variant”.
References
Allen, RS, Tajer B, Shore EM, and Mullins MC. Fibrodysplasia ossificans progressiva mutant ACVR1 signals by multiple modalities in the developing zebrafish. eLife 9:e53761, 2020
Haupt J, Xu M, Shore EM. Variable signaling activity by FOP ACVR1mutations. Bone 109: 232-240, 2018
Huning I & Gillessen-Kaesbach. Fibrodysplasia Ossificans Progressiva: Clinical Course, Genetic Mutations and Genotype-Phenotype Correlations. Molec Syndromology 5: 201-211, 2014
Kaplan FS, Groppe JC, Xu M, Towler OW, Grunvald E, Kalunian K, Kallish S, Al Mukaddam M, Pignolo RJ, Shore EM. An ACVR1R375P pathogenic variant in two families with mild fibrodysplasia ossificans progressiva. Am J Med Genet A 188: 806-817, 2022
Kaplan FS, Kobori JA, Orellana C, Calvo I, Rosello M, Martinez F, Lopez B, Xu M, Pignolo RJ, Shore EM, Groppe JC. Multi-system involvement in a severe variant of fibrodysplasia ossificans progressiva (ACVR1c.772G>A; R258G): a report of two patients. Am J Med Genetic A 167: 2265-2271, 2015
Kaplan FS, Xu M, Seemann P, Connor M, Glaser DL, Carroll L, Delai, P, Fastnact-Urban E, Forman SJ, Gillessen-Kaesbach G, Hoover-Fong J, Köster B, Morhart R, Pauli RM, Reardon W, Zaidi SA, Zasloff M, Mundlos S, Groppe J, Shore EM. Classical and atypical FOP phenotypes are caused by mutations in the BMP type I receptor ACVR1. Human Mutation 30: 379-390, 2009
Mucha BE, Hashiguchi M, Zinski J, Shore EM, Mullins MC. Variant BMP receptor mutations causing fibrodysplasia ossificans progressiva (FOP) in humans show BMP ligand-independent receptor activation in zebrafish. Bone 109: 225-231, 2018
5-37. Impact of FOP on Patients and Family
A recent international burden of illness survey (NCT04665323) assessed physical, quality of life (QoL), and economic impacts of FOP on patients and family members (Al Mukaddam et al., 2022). Patient associations in 15 countries invited their members to participate; individuals with FOP and their family members were eligible. The survey was available online, in 11 languages, from 18 January-30 April 2021. Participants responded to assessments measuring joint function, QoL, healthcare service and living adaptation utilization, out-of-pocket costs, employment, and travel.
The survey received 463 responses (patients, n = 219; family members, n = 244). For patients, decreased joint function was associated with reduced QoL and greater reliance on living adaptations. Nearly half of primary caregivers experienced a mild to moderate impact on their health/psychological wellbeing. Most primary caregivers and patients (≥18 years) reported that FOP impacted their career decisions. Data from this survey will aid understanding of the impact of FOP on patients and family members as well as identifying unmet needs, optimizing care, and improving support for the FOP community.
References
Al Mukaddam M, Toder KS, Davis M, Cali A, Liljesthröm M, Hollywood S, Croskery K, Grandoulier AS, Böing EA, Whalen JD, Kaplan FS. The impact of fibrodysplasia ossificans progressiva (FOP) on patients and their family members: results from an international burden of illness survey. Expert Rev Pharmacoecon Outcomes Res 22: 1199-1213, 2022
5-38. Unmet Needs in FOP
Assiduous attention to the unmet needs of individuals with FOP is crucial to prevent potential iatrogenic harm and optimize care for individuals with FOP. Individuals with FOP often face the frustration of long diagnostic journeys, the burden of self-advocacy and the navigation of novel care pathways.
Globally, patients with FOP are also confronted with inequities in access to diagnosis and specialist care, and consequently, unequal access to registries, clinical trials, and essential support from patient associations. Organizations such as the International Fibrodysplasia Ossificans Progressiva Association (IFOPA; www.ifopa.org), the International Clinical Council on FOP (ICC; www.iccfop.org), and national FOP organizations work to provide information, facilitate access to expert clinical guidance, nurture patient empowerment, fund FOP research and/or foster meaningful collaborations with the research community.
The non-profit Tin Soldiers Global FOP Patient Search program aims to identify and provide a pathway to diagnosis and care for individuals with FOP, particularly in underserved communities.
Such global initiatives and the increasingly widespread use of telemedicine and digital platforms offer opportunities to improve vital access to care and research. Regional and international organizations play an important role in improving the quality of life of those they reach in the global FOP community. However, globally, fundamental issues remain around raising awareness of FOP among healthcare professionals, identifying individuals with FOP, reducing time to diagnosis, and ensuring access to best practice in care, support, and clinical research (Pignolo, Bedford-Gay et al., 2022).
References
Pignolo RJ, Bedford-Gay C, Cali A, Davis M, Delai PLR, Gonzales K, Hixson C, Kent A, Newport H, Robert M, Scott C, Kaplan FS. Current challenges and opportunities in the care of patients with fibrodysplasia ossificans progressiva (FOP): an international, multi-stakeholder perspective. Orphanet J Rare Dis 17:168, 2022